92549
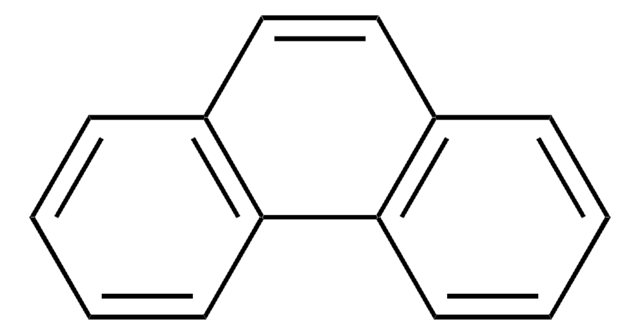
Acenaphthylene
certified reference material, TraceCERT®, Manufactured by: Sigma-Aldrich Production GmbH, Switzerland
About This Item
Prodotti consigliati
Grado
certified reference material
TraceCERT®
Livello qualitativo
Nome Commerciale
TraceCERT®
Durata
limited shelf life, expiry date on the label
Produttore/marchio commerciale
Manufactured by: Sigma-Aldrich Production GmbH, Switzerland
tecniche
HPLC: suitable
gas chromatography (GC): suitable
P. ebollizione
280 °C (lit.)
Punto di fusione
78-82 °C (lit.)
89-92 °C
Densità
0.899 g/mL at 25 °C (lit.)
applicazioni
environmental
Formato
neat
Temperatura di conservazione
2-8°C
Stringa SMILE
c1cc2C=Cc3cccc(c1)c23
InChI
1S/C12H8/c1-3-9-4-2-6-11-8-7-10(5-1)12(9)11/h1-8H
HXGDTGSAIMULJN-UHFFFAOYSA-N
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Descrizione generale
Certified content by quantitative NMR incl. uncertainty and expiry date are given on the certificate.
Download your certificate at: http://www.sigma-aldrich.com.
Applicazioni
Confezionamento
Note legali
Avvertenze
Warning
Indicazioni di pericolo
Consigli di prudenza
Classi di pericolo
Acute Tox. 4 Oral - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Organi bersaglio
Respiratory system
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
251.6 °F - closed cup
Punto d’infiammabilità (°C)
122.0 °C - closed cup
Scegli una delle versioni più recenti:
Certificati d'analisi (COA)
It looks like we've run into a problem, but you can still download Certificates of Analysis from our Documenti section.
Se ti serve aiuto, non esitare a contattarci Servizio Clienti
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
Protocolli
US EPA Method 8270 (PAH only): GC Analysis of PAHs on SLB®-5ms
US EPA Method 610 describes the analysis of polynuclear aromatic hydrocarbons (commonly referred to as PAHs or PNAs) by both HPLC and GC.
GC Analysis of Polynuclear Aromatic Hydrocarbons (PAHs) in Salmon on SPB®-608 (20 m x 0.18 mm I.D., 0.18 µm) after QuEChERS Cleanup using Supel™ QuE Z-Sep, Fast GC Analysis
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.

![[(3R)-3-Hydroxydecanoyl]-L-carnitine-(N-methyl-d3) analytical standard](/deepweb/assets/sigmaaldrich/product/structures/273/425/010200db-ebc0-4746-b60c-4ac5029b9958/640/010200db-ebc0-4746-b60c-4ac5029b9958.png)