426288
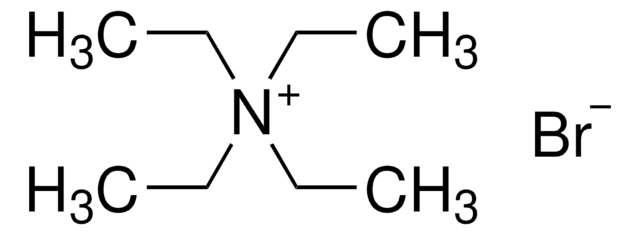
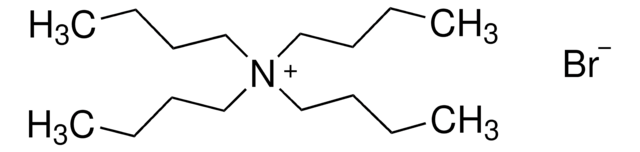
Tetrabutylammonium bromide
ACS reagent, ≥98.0%
Sinonimo/i:
N,N,N-tributyl-1-butanaminium bromide, TBAB, TBABr, tetra-n-butylammonium bromide
About This Item
Prodotti consigliati
Grado
ACS reagent
Livello qualitativo
Saggio
≥98.0%
Forma fisica
solid
Caratteristiche più verdi
Catalysis
Learn more about the Principles of Green Chemistry.
sustainability
Greener Alternative Product
Impurezze
≤0.5% tributylamine hydrobromide
≤0.5% tributylamine
Punto di fusione
102-106 °C (lit.)
Gruppo funzionale
amine
Categoria alternativa più verde
Stringa SMILE
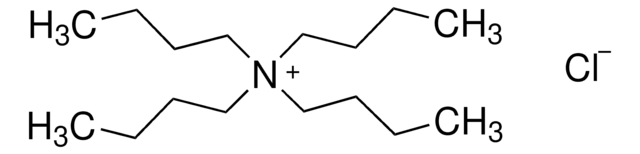
[Br-].CCCC[N+](CCCC)(CCCC)CCCC
InChI
1S/C16H36N.BrH/c1-5-9-13-17(14-10-6-2,15-11-7-3)16-12-8-4;/h5-16H2,1-4H3;1H/q+1;/p-1
JRMUNVKIHCOMHV-UHFFFAOYSA-M
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Descrizione generale
Applicazioni
- Synthesis of (2S)-5-(3-phenyl-2-phthalimidylpropanoylamino)isophthalic acid.
- Synthesis of alkyl-substituted pyrroles in the absence of catalyst and organic solvent.
- Synthesis of dithioacetals from acetals by transthioacetalisation in a solvent free environment.
- Synthesis of polyamides (PAs) by the polymerization of terephthalic acid and diisocyanates.
- Catalyze the addition of thiols to conjugated alkenes.
- Dehydrochlorination of poly(vinyl chloride).
Process for Producing Halogenated Heteroaryl Compounds
Avvertenze
Warning
Indicazioni di pericolo
Consigli di prudenza
Classi di pericolo
Acute Tox. 4 Oral - Aquatic Chronic 3 - Eye Irrit. 2 - Repr. 2 - Skin Irrit. 2
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Dispositivi di protezione individuale
dust mask type N95 (US), Eyeshields, Gloves
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.
![4-[4-(Dimethylamino)styryl]pyridine 95%](/deepweb/assets/sigmaaldrich/product/structures/225/605/ad18cc93-9d43-467b-8618-105948f9692b/640/ad18cc93-9d43-467b-8618-105948f9692b.png)

![trans-4-[4-(Dimethylamino)styryl]-1-methylpyridinium iodide Dye content 98 %](/deepweb/assets/sigmaaldrich/product/structures/416/722/5d59b6c3-5f2d-4396-a721-5cb82ba7038c/640/5d59b6c3-5f2d-4396-a721-5cb82ba7038c.png)