31102
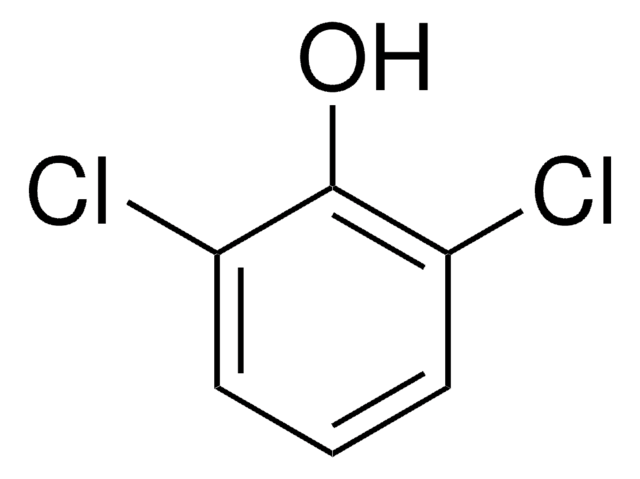
2,6-Dichlorophenol
PESTANAL®, analytical standard
About This Item
Prodotti consigliati
Grado
analytical standard
Nome Commerciale
PESTANAL®
Durata
limited shelf life, expiry date on the label
tecniche
HPLC: suitable
gas chromatography (GC): suitable
P. ebollizione
218-220 °C (lit.)
Punto di fusione
64-66 °C (lit.)
applicazioni
agriculture
environmental
Formato
neat
Stringa SMILE
Oc1c(Cl)cccc1Cl
InChI
1S/C6H4Cl2O/c7-4-2-1-3-5(8)6(4)9/h1-3,9H
HOLHYSJJBXSLMV-UHFFFAOYSA-N
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Categorie correlate
Descrizione generale
Applicazioni
- To study the kinetics, performance, and mechanism of the oxidative degradation of 2,6-dichlorophenol (2,6-DCP) by ferrate (VI) (Fe(VI))
- Removal of 2,6-dichlorophenol in water by copper oxide (CuO) activated peroxymonosulfate as catalyst
- Removal of 2,6-dichlorophenol by adsorption with activated polypropylene nanofiber
- Degradation of 2,6-dichlorophenol by Fe-doped titanium oxide(TiO2) sonophotocatalytic process
- Determination of 2,6-dichlorophenol by surface-enhanced Raman scattering (SERS) with molecular imprinting
Note legali
Avvertenze
Danger
Indicazioni di pericolo
Classi di pericolo
Skin Corr. 1B
Codice della classe di stoccaggio
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
Classe di pericolosità dell'acqua (WGK)
WGK 2
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Dispositivi di protezione individuale
Eyeshields, Faceshields, Gloves, type P3 (EN 143) respirator cartridges
Scegli una delle versioni più recenti:
Certificati d'analisi (COA)
Non trovi la versione di tuo interesse?
Se hai bisogno di una versione specifica, puoi cercare il certificato tramite il numero di lotto.
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.