17755
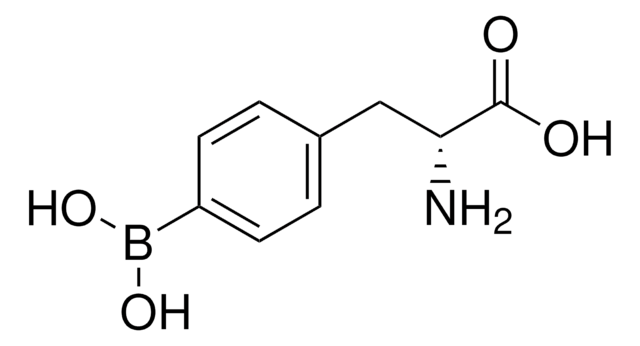
4-Borono-L-phenylalanine
≥95.0% (HPLC)
Sinonimo/i:
4-Dihydroxyboryl-L-phenylalanine, L-BPA
Autenticatiper visualizzare i prezzi riservati alla tua organizzazione & contrattuali
About This Item
Formula empirica (notazione di Hill):
C9H12BNO4
Numero CAS:
Peso molecolare:
209.01
Beilstein:
4458616
Numero MDL:
Codice UNSPSC:
12352209
ID PubChem:
NACRES:
NA.21
Prodotti consigliati
Livello qualitativo
Saggio
≥95.0% (HPLC)
Temperatura di conservazione
2-8°C
Stringa SMILE
N[C@@H](Cc1ccc(cc1)B(O)O)C(O)=O
InChI
1S/C9H12BNO4/c11-8(9(12)13)5-6-1-3-7(4-2-6)10(14)15/h1-4,8,14-15H,5,11H2,(H,12,13)/t8-/m0/s1
NFIVJOSXJDORSP-QMMMGPOBSA-N
Applicazioni
4-Borono-L-phenylalanine can be used as a building block in solid-phase peptide synthesis. It can also be used to synthesize substituted triazine derivatives as potential tryptophan hydroxylase inhibitors via Suzuki cross-coupling reaction using palladium as a catalyst.
Altre note
Tyrosine analogue; employed for treatment of melanom cells by boron neutron capture therapy
Avvertenze
Warning
Indicazioni di pericolo
Consigli di prudenza
Classi di pericolo
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Organi bersaglio
Respiratory system
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Dispositivi di protezione individuale
dust mask type N95 (US), Eyeshields, Gloves
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
Romina F Aromando et al.
Oral oncology, 46(5), 355-359 (2010-03-24)
Mast cell (MC) activation in the hamster cheek pouch cancerization model is associated with the increase in tumor cell proliferation, mediated in turn by tryptase, a protease released from mast cell granules after activation. Tryptase induces tumor cell proliferation through
T Fujimoto et al.
Applied radiation and isotopes : including data, instrumentation and methods for use in agriculture, industry and medicine, 69(12), 1713-1716 (2011-03-01)
Clear cell sarcoma (CCS), a rare malignant tumor with a predilection for young adults, is of poor prognosis. Recently however, boron neutron capture therapy (BNCT) with the use of p-borono-L-phenylalanine (BPA) for malignant melanoma has provided good results. CCS also
Ana J Molinari et al.
Radiation research, 177(1), 59-68 (2011-10-11)
We previously demonstrated the efficacy of BNCT mediated by boronophenylalanine (BPA) to treat tumors in a hamster cheek pouch model of oral cancer with no normal tissue radiotoxicity and moderate, albeit reversible, mucositis in precancerous tissue around treated tumors. It
Andrea Wittig et al.
Radiation research, 172(4), 493-499 (2009-09-24)
In boron neutron capture therapy, the absorbed dose from the (10)B(n,alpha)(7)Li reaction depends on the (10)B concentration and (10)B distribution in the irradiated volume. Thus compounds used in BNCT should have tumor-specific uptake and low accumulation in normal tissues. This
Tsubasa Watanabe et al.
BMC cancer, 16(1), 859-859 (2016-11-09)
Boron neutron capture therapy (BNCT) is a cellular-level particle radiation therapy that combines the selective delivery of boron compounds to tumour tissue with neutron irradiation. L-p-Boronophenylalanine (L-BPA) is a boron compound now widely used in clinical situations. Determination of the
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.