MAB8661
Anti-Influenza B Antibody, nucleoprotein, clones B2, B4 Blend
ascites fluid, Chemicon®, from mouse
Autenticatiper visualizzare i prezzi riservati alla tua organizzazione & contrattuali
About This Item
Codice UNSPSC:
12352203
eCl@ss:
32160702
NACRES:
NA.41
Prodotti consigliati
Origine biologica
mouse
Livello qualitativo
Forma dell’anticorpo
ascites fluid
Tipo di anticorpo
primary antibodies
Clone
B2, monoclonal
B4, monoclonal
Reattività contro le specie
human
Produttore/marchio commerciale
Chemicon®
tecniche
immunofluorescence: suitable
Isotipo
IgG
Condizioni di spedizione
wet ice
Specificità
Recognizes all subtypes of Influenza B. Blend of two clones that recognize the nucleoprotein antigen.
No cross-reactivity to influenza A, parainfluenza 1, 2 or 3, adenovirus or RSV.
No cross-reactivity to influenza A, parainfluenza 1, 2 or 3, adenovirus or RSV.
Immunogeno
Influenza Blend.
Applicazioni
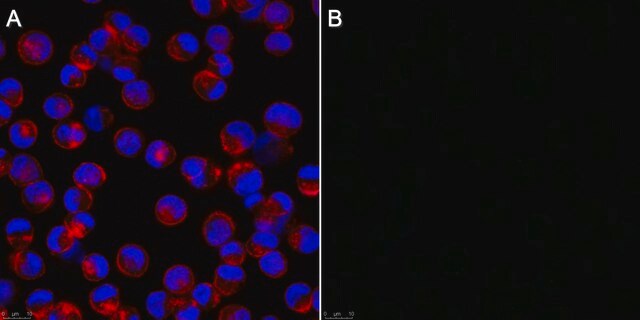
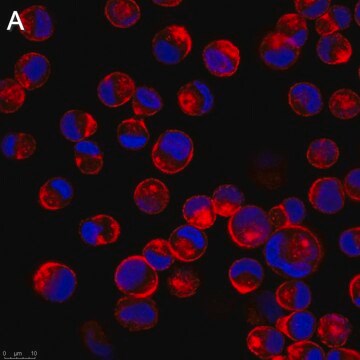
Anti-Influenza B Antibody, nucleoprotein, clones B2, B4 Blend detects level of Influenza B & has been published & validated for use in IF.
Indirect immunofluorescence at 1:100.
Final working dilutions must be determined by end user.
Final working dilutions must be determined by end user.
Research Category
Infectious Diseases
Infectious Diseases
Research Sub Category
Infectious Diseases - Viral
Infectious Diseases - Viral
Stato fisico
Ascites fluid with 0.1% sodium azide as a preservative.
Unpurified
Stoccaggio e stabilità
Maintain for 1 year at -20°C from date of shipment. Aliquot to avoid repeated freezing and thawing. For maximum recovery of product, centrifuge the original vial after thawing and prior to removing the cap.
Risultati analitici
Control
Influenza Control Slides, Catalogue Number 5010-5
Influenza Control Slides, Catalogue Number 5010-5
Altre note
Concentration: Please refer to the Certificate of Analysis for the lot-specific concentration.
Note legali
CHEMICON is a registered trademark of Merck KGaA, Darmstadt, Germany
Esclusione di responsabilità
Unless otherwise stated in our catalog or other company documentation accompanying the product(s), our products are intended for research use only and are not to be used for any other purpose, which includes but is not limited to, unauthorized commercial uses, in vitro diagnostic uses, ex vivo or in vivo therapeutic uses or any type of consumption or application to humans or animals.
Non trovi il prodotto giusto?
Prova il nostro Motore di ricerca dei prodotti.
Codice della classe di stoccaggio
12 - Non Combustible Liquids
Classe di pericolosità dell'acqua (WGK)
nwg
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Certificati d'analisi (COA)
Cerca il Certificati d'analisi (COA) digitando il numero di lotto/batch corrispondente. I numeri di lotto o di batch sono stampati sull'etichetta dei prodotti dopo la parola ‘Lotto’ o ‘Batch’.
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
Genetic variability of human metapneumovirus isolated from Chilean children, 2003-2004.
Carola Escobar,Vivian Luchsinger,Danielle Bruna de Oliveira,Edison Durigon et al.
Journal of Medical Virology null
Kevin R McCarthy et al.
Proceedings of the National Academy of Sciences of the United States of America, 118(23) (2021-06-03)
Immune memory of a first infection with influenza virus establishes a lasting imprint. Recall of that memory dominates the response to later infections or vaccinations by antigenically drifted strains. Early childhood immunization before infection may leave an imprint with different
Aaron G Schmidt et al.
Cell, 161(5), 1026-1034 (2015-05-12)
Vaccines for rapidly evolving pathogens will confer lasting immunity if they elicit antibodies recognizing conserved epitopes, such as a receptor-binding site (RBS). From characteristics of an influenza-virus RBS-directed antibody, we devised a signature motif to search for similar antibodies. We
Paulina Koszalka et al.
Antiviral research, 164, 91-96 (2019-02-17)
Baloxavir Marboxil (BXM) is an influenza polymerase inhibitor antiviral that binds to the endonuclease region in the PA subunit of influenza A and B viruses. To establish the baseline susceptibility of viruses circulating prior to licensure of BXM and to
Tina Schmidt et al.
European journal of immunology, 42(7), 1755-1766 (2012-05-16)
Antigen-specific antibodies are well characterized after vaccination with pandemic H1N1 or seasonal influenza vaccines. However, knowledge on cellular immunity toward pandemic H1N1 after vaccination and infection and cross-reactivities toward seasonal antigens is limited. Nineteen individuals were vaccinated with the pandemic
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.