8.52079
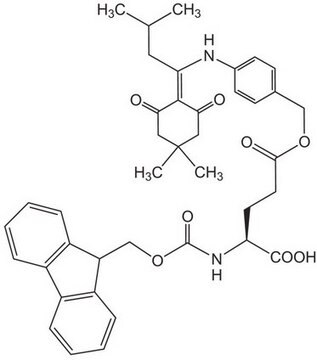
Fmoc-Asp-ODmab
Novabiochem®
Sinonimo/i:
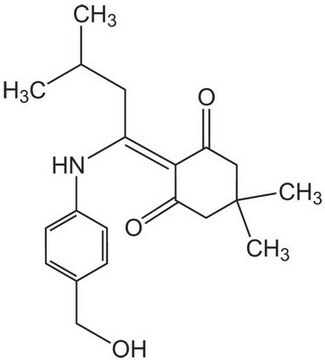
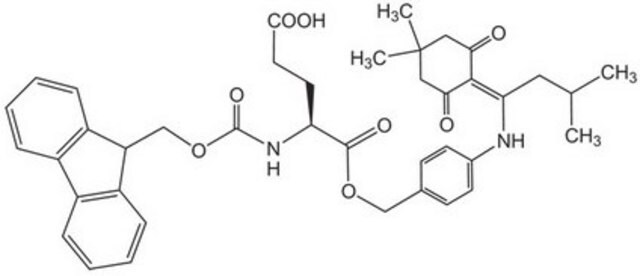
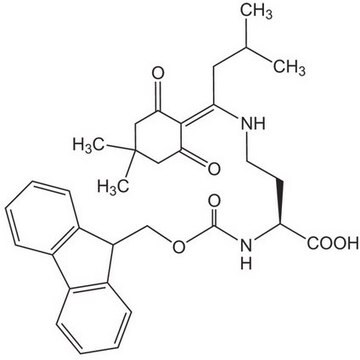
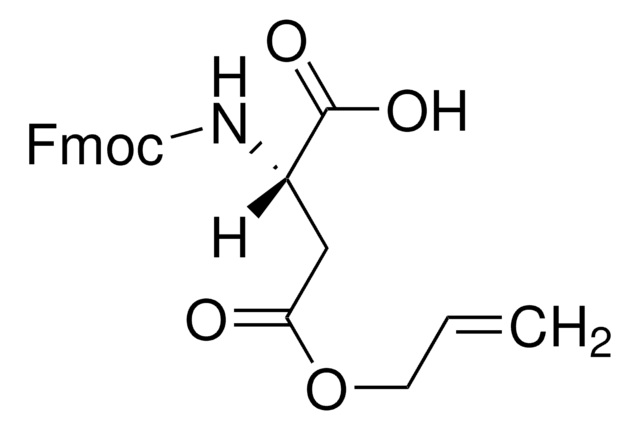
Fmoc-Asp-ODmab, N-α-Fmoc-L-aspartic acid α-4-{N-[1-(4,4-dimethyl-2,6-dioxocyclohexylidene)-3-methylbutyl]-amino} benzyl ester
About This Item
Prodotti consigliati
Livello qualitativo
Nome Commerciale
Novabiochem®
Saggio
≥95.0% (HPLC)
≥97% (TLC)
Stato
powder
Impiego in reazioni chimiche
reaction type: Fmoc solid-phase peptide synthesis
Produttore/marchio commerciale
Novabiochem®
applicazioni
peptide synthesis
Gruppo funzionale
carboxylic acid
Temperatura di conservazione
15-25°C
Stringa SMILE
N([C@@H](CC(=O)O)C(=O)OCc4ccc(cc4)NC(=C5C(=O)CC(CC5=O)(C)C)CC(C)C)C(=O)OCC1c2c(cccc2)c3c1cccc3
InChI
1S/C39H42N2O8/c1-23(2)17-31(36-33(42)19-39(3,4)20-34(36)43)40-25-15-13-24(14-16-25)21-48-37(46)32(18-35(44)45)41-38(47)49-22-30-28-11-7-5-9-26(28)27-10-6-8-12-29(27)30/h5-16,23,30,32,40H,17-22H2,1-4H3,(H,41,47)(H,44,45)/t32-/m0/s1
QPTKEHUFSCXXEH-YTTGMZPUSA-N
Descrizione generale
Associated Protocols and Technical Articles
Cleavage and Deprotection Protocols for Fmoc SPPS
Literature references
[1] W. C. Chan, et al. (1995) J. Chem. Soc., Chem. Commun., 2209.
[2] S. Künzel, et al. Poster 17 presented at Solid Phase Synthesis & Combinatorial Libraries, Southampton, September 2001.
[3] K. F. Medzihradszky, et al. (2002) Lett. Pept. Sci., 8, 1.
[4] Albericio, et al.Poster 44 presented at American Peptide Symposium, San Diego 2005..
[5] M. Cudic, et al. in ′Peptides 2000, Proc. 26th European Peptide Symposium′, J. Martinez & J.-A. Fehrentz (Eds), Paris, Editions EDK, 2001, pp. 203.
[6] M. Cudic, et al. (2000) Tetrahedron Lett., 41, 4527.
[7] J. P. Malkinson, et al. (2003) Org. Lett., 5, 5051.
Linkage
Risultati analitici
Appearance of substance (visual): powder
Identity (IR): passes test
Enantiomeric purity: ≥ 99.0 % (a/a)
Purity (TLC(157A)): ≥ 97 %
Purity (TLC(CMA2)): ≥ 97 %
Assay (HPLC, area%): ≥ 95.0 % (a/a)
Solubility (1 mmole in 2 ml DMF): clearly soluble
Water (K. F.): ≤ 1.0 %
To see the solvent systems used for TLC of Novabiochem® products please click here.
Note legali
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 2
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Certificati d'analisi (COA)
Cerca il Certificati d'analisi (COA) digitando il numero di lotto/batch corrispondente. I numeri di lotto o di batch sono stampati sull'etichetta dei prodotti dopo la parola ‘Lotto’ o ‘Batch’.
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
Articoli
Novabiochem® product range has one of the largest collections of orthogonally and quasi-orthogonally protected tri-functional amino acids. These derivatives are useful tools for the synthesis of cyclic and branched peptides and peptides carrying side-chain modifications.
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.