1.46429
Agar di soia triptico - Flaconi & provette pronte all′uso
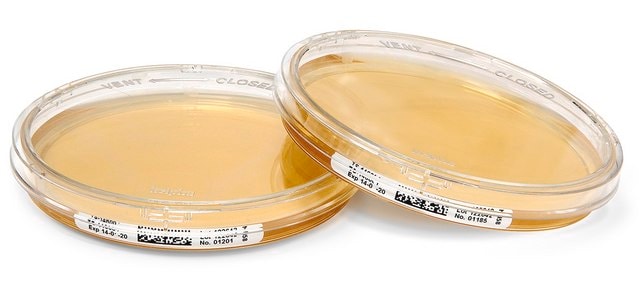
tube capacity 25 mL, tube filling volume 18 mL, closure type, blue screw cap with septum, box of 20 or 100 tubes
Sinonimo/i:
CASO Agar, Casein Soybean Digest Agar, TSA, Tryptic Soy Agar, Tryptone Soya Agar
About This Item
Prodotti consigliati
agenzia
EP 2.6.13
JP 4.05
USP 62
Livello qualitativo
Descrizione
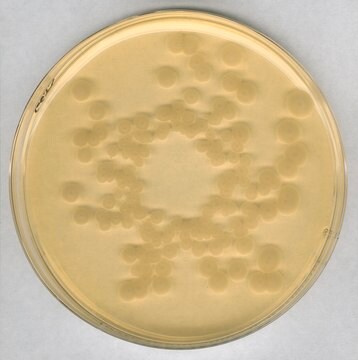
For microbiological examination of non-sterile products
Sterilità
sterile
Forma fisica
liquid (ready-to-use)
Durata
12 mo.
Caratteristiche
closure type blue screw cap with septum
ready-to-use
Confezionamento
box of 20 or 100 tubes
single packed of (18 ml in 25 ml tube with blue cap (20 or 100 tubes per box))
tecniche
direct inoculation: suitable
sterile filtration: suitable
Capacità
25 mL
tube filling volume
18 mL
Colore
clear yellowish
pH
7.3
applicazioni
bioburden testing
cosmetics
food and beverages
pharmaceutical
Temperatura di conservazione
2-25°C
Descrizione generale
Applicazioni
Caratteristiche e vantaggi
- Our ready-to-use media provide the highest level of quality and testing confidence. They have been formulated and tested to meet the pharmacopoeia requirements.
- Sterility testing media and rinse solutions are manufactured in an ISO 9001, environmentally controlled production center. Each lot undergoes a stringent quality control (QC) procedure, including pH, sterility, and growth promotion tests.
- Our manufacturing approach ensures the highest level of clarity for our media and rinsing fluids, therefore improving accuracy, and significantly reducing the risk of incorrect interpretation and false results.
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 1
Certificati d'analisi (COA)
Cerca il Certificati d'analisi (COA) digitando il numero di lotto/batch corrispondente. I numeri di lotto o di batch sono stampati sull'etichetta dei prodotti dopo la parola ‘Lotto’ o ‘Batch’.
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.