860820P
Avanti
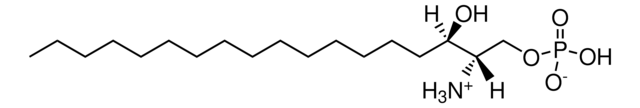
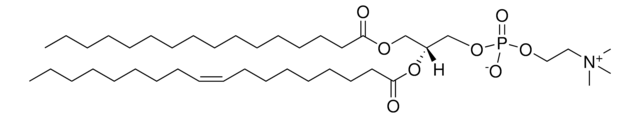
20:0(2S-OH) Ceramide
Avanti Polar Lipids 860820P, powder
Sinonimo/i:
N-(2′-(S)-hydroxyarachidoyl)-D-erythro-sphingosine
Autenticatiper visualizzare i prezzi riservati alla tua organizzazione & contrattuali
About This Item
Formula empirica (notazione di Hill):
C38H75NO4
Numero CAS:
Peso molecolare:
610.01
Codice UNSPSC:
12352211
NACRES:
NA.25
Prodotti consigliati
Forma fisica
powder
Confezionamento
pkg of 1 × 5 mg (860820P-5mg)
Produttore/marchio commerciale
Avanti Polar Lipids 860820P
Tipo di lipide
sphingolipids
Condizioni di spedizione
dry ice
Temperatura di conservazione
−20°C
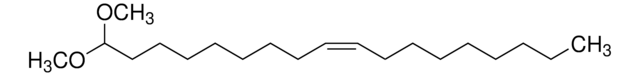
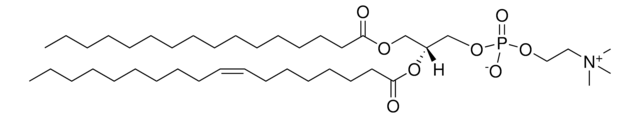
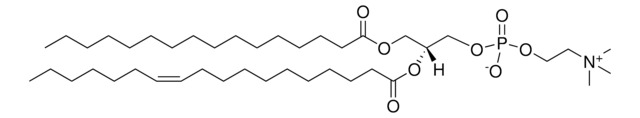
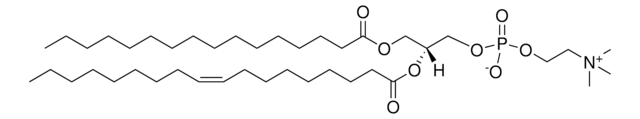
Stringa SMILE
CCCCCCCCCCCCC/C=C/[C@@H](O)[C@@H](NC([C@@H](O)CCCCCCCCCCCCCCCCCC)=O)CO
Categorie correlate
Descrizione generale
Ceramides linked to 2-hydroxy fatty acids (hFA) are present in the surface epithelium of the skin. 20:0(2S-OH) Ceramide is a unique ceramide containing 20C long chain base fatty acid (arachidic acid)-with 2′-hydroxyl group in S configuration.
Azioni biochim/fisiol
Hydroxy fatty acid (hFA)-sphingolipids help in formation and function of myelin. In addition, they also play a vital role in cell signaling, cell differentiation and apoptosis. In epidermis, hFA-ceramides aid in permeability barrier function. NAD(P)H-dependent enzyme, fatty acid 2-hydroxylase (FA2H) catalyzes the synthesis of hFA-ceramides. FA2H gene mutation leads to the development of neurological disorders such as leukodystrophy and spastic paraparesis in humans. hFA-ceramides help PM02734 (elisidepsin), an antitumor drug to exhibit its activity.
Confezionamento
5 mL Amber Glass Screw Cap Vial (860820P-5mg)
Note legali
Avanti Research is a trademark of Avanti Polar Lipids, LLC
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Certificati d'analisi (COA)
Cerca il Certificati d'analisi (COA) digitando il numero di lotto/batch corrispondente. I numeri di lotto o di batch sono stampati sull'etichetta dei prodotti dopo la parola ‘Lotto’ o ‘Batch’.
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
Yi-He Ling et al.
Clinical cancer research : an official journal of the American Association for Cancer Research, 17(16), 5353-5366 (2011-06-22)
PM02734 (elisidepsin) is a synthetic marine-derived cyclic peptide of the kahalalide family currently in phase II clinical development. The mechanisms of cell death induced by PM02734 remain unknown. Human non-small-cell lung cancer (NSCLC) cell lines H322 and A549 were used
PM02734 (elisidepsin) induces caspase-independent cell death associated with features of autophagy, inhibition of the Akt/mTOR signaling pathway, and activation of death-associated protein kinase
Ling YH, et al.
Clinical Cancer Research, 17(16), 5353-5366 (2011)
Normal fur development and sebum production depends on fatty acid 2-hydroxylase expression in sebaceous glands
Maier H, et al.
The Journal of Biological Chemistry, 286(29), 25922-25934 (2011)
Fatty acid 2-Hydroxylation in mammalian sphingolipid biology
Hama H, et al.
Biochimica et Biophysica Acta - Molecular and Cell Biology of Lipids, 1801(4), 405-414 (2010)
Ana B Herrero et al.
Cancer research, 68(23), 9779-9787 (2008-12-03)
PM02734 is a novel synthetic antitumor drug that is currently in phase I clinical trials. To gain some insight into its mode of action, we used the yeast Saccharomyces cerevisiae as a model system. Treatment of S. cerevisiae with PM02734
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.