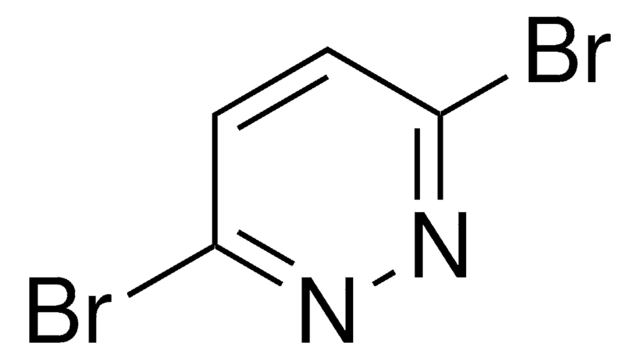
P57204
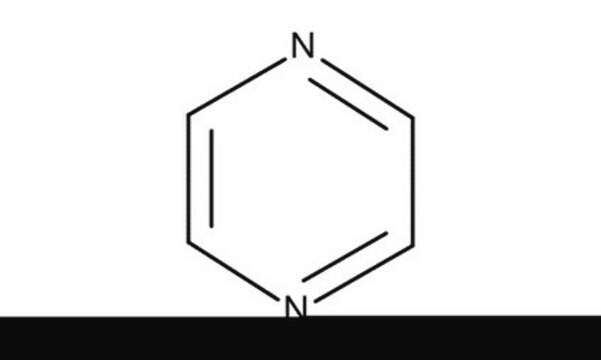
Pyridazine
98%
Sinonimo/i:
1,2-Diazabenzene, 1,2-Diazine, o-Diazine
Autenticatiper visualizzare i prezzi riservati alla tua organizzazione & contrattuali
About This Item
Formula empirica (notazione di Hill):
C4H4N2
Numero CAS:
Peso molecolare:
80.09
Beilstein:
103906
Numero CE:
Numero MDL:
Codice UNSPSC:
12352100
ID PubChem:
NACRES:
NA.22
Prodotti consigliati
Saggio
98%
Indice di rifrazione
n20/D 1.524 (lit.)
P. ebollizione
208 °C (lit.)
Punto di fusione
−8 °C (lit.)
Densità
1.103 g/mL at 25 °C (lit.)
Stringa SMILE
c1ccnnc1
InChI
1S/C4H4N2/c1-2-4-6-5-3-1/h1-4H
PBMFSQRYOILNGV-UHFFFAOYSA-N
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Descrizione generale
Pyridazine is a mono-basic 1,2-diazine compound, which is commonly prepared by the reaction of 1,4-dicarbonyls with hydrazines. Pyridazine ring is found in many herbicides like credazine, pyridatol and many pharmaceutical drugs like cefozopran, olaparib, talazoparib, and cadralazine.
Applicazioni
Pyridazine can be used:
- As a building block to synthesize N-phenyl-4-pyrazolo[1,5-b]pyridazin-3-yl-pyrimidin-2-amine derivatives as GSK-3 inhibitors.
- As a starting material in the synthesis of pharmacologically important pyrrolo[1,2-b]pyridazine derivatives.
- To prepare 1-(6-ethoxy-6-oxohexyl)pyridazin-1-ium bromide and 1-(2-bromoacetyl) pyridazinium bromide ionic liquids as corrosion inhibitors of steel under acidic conditions.
Codice della classe di stoccaggio
10 - Combustible liquids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
185.0 °F - closed cup
Punto d’infiammabilità (°C)
85 °C - closed cup
Dispositivi di protezione individuale
Eyeshields, Gloves, multi-purpose combination respirator cartridge (US)
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Masaomi Terajima et al.
European journal of pharmacology, 698(1-3), 455-462 (2012-11-28)
Given the key role p38 mitogen-activated protein kinase (MAPK) plays in inflammatory responses through the production of cytokines and inflammatory mediators, its inhibition is considered a promising therapeutic strategy for chronic inflammatory diseases such as rheumatoid arthritis, psoriasis, inflammatory bowel
Jeffrey R Reimers et al.
Physical chemistry chemical physics : PCCP, 14(25), 8791-8802 (2012-04-26)
A unified picture is presented of water interacting with pyridine, pyridazine, pyrimidine, and pyrazine on the S(1) manifold in both gas-phase dimers and in aqueous solution. As (n,π*) excitation to the S(1) state removes electrons from the ground-state hydrogen bond
Emily A Peterson et al.
Bioorganic & medicinal chemistry letters, 22(15), 4967-4974 (2012-07-07)
mTOR is a critical regulator of cellular signaling downstream of multiple growth factors. The mTOR/PI3K/AKT pathway is frequently mutated in human cancers and is thus an important oncology target. Herein we report the evolution of our program to discover ATP-competitive
Six membered heterocyclic compounds with two or more heteroatoms
Heterocyclic Chemistry (2010)
Alessio Raimondi et al.
Inorganic chemistry, 51(5), 2966-2975 (2012-03-01)
A series of [Re(2)(μ-ER)(2)(CO)(6)(μ-pydz)] complexes have been synthesized (E = S, R = C(6)H(5), 2; E = O, R = C(6)F(5), 3; C(6)H(5), 4; CH(3), and 5; H, 6), starting either from [Re(CO)(5)O(3)SCF(3)] (for 2 and 4), [Re(2)(μ-OR)(3)(CO)(6)](-) (for 3
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.

![1,8-diazabiciclo[5.4.0]undec-7-ene 98%](/deepweb/assets/sigmaaldrich/product/structures/120/564/5b373e23-1624-489c-8efb-692de0f96ffb/640/5b373e23-1624-489c-8efb-692de0f96ffb.png)