P22370
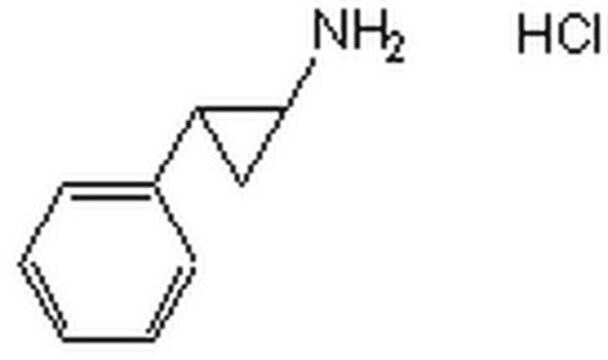
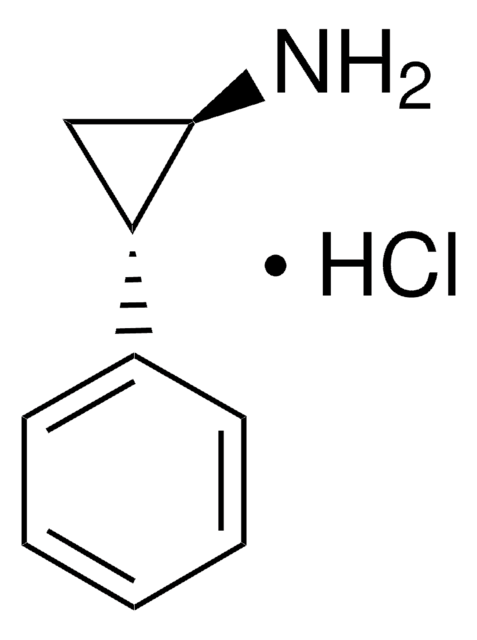
trans-2-Phenylcyclopropylamine hydrochloride
97%
Sinonimo/i:
Tranylcypromine
Autenticatiper visualizzare i prezzi riservati alla tua organizzazione & contrattuali
About This Item
Formula condensata:
C6H5C3H4NH2·HCl
Numero CAS:
Peso molecolare:
169.65
Numero MDL:
Codice UNSPSC:
12352100
ID PubChem:
NACRES:
NA.22
Prodotti consigliati
Saggio
97%
Stato
solid
Attività ottica
[α]/D −1 to +1.0°, c = 1 in H2O
Punto di fusione
162-169 °C (lit.)
Temperatura di conservazione
2-8°C
Stringa SMILE
Cl.N[C@@H]1C[C@H]1c2ccccc2
InChI
1S/C9H11N.ClH/c10-9-6-8(9)7-4-2-1-3-5-7;/h1-5,8-9H,6,10H2;1H/t8-,9+;/m0./s1
ZPEFMSTTZXJOTM-OULXEKPRSA-N
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Categorie correlate
Azioni biochim/fisiol
Non-selective MAO-A/B inhibitor.
Avvertenze
Danger
Indicazioni di pericolo
Consigli di prudenza
Classi di pericolo
Acute Tox. 3 Oral
Codice della classe di stoccaggio
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
Classe di pericolosità dell'acqua (WGK)
WGK 3
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
Kaisa A Salminen et al.
Drug metabolism and disposition: the biological fate of chemicals, 43(12), 1891-1904 (2015-09-25)
The cytochrome P450 2C19 (CYP2C19) enzyme plays an important role in the metabolism of many commonly used drugs. Relatively little is known about CYP2C19 inhibitors, including compounds of natural origin, which could inhibit CYP2C19, potentially causing clinically relevant metabolism-based drug
James M Hill et al.
Science translational medicine, 6(265), 265ra169-265ra169 (2014-12-05)
Herpesviruses are highly prevalent and maintain lifelong latent reservoirs, thus posing challenges to the control of herpetic disease despite the availability of antiviral pharmaceuticals that target viral DNA replication. The initiation of herpes simplex virus infection and reactivation from latency
Ramakrishna Nirogi et al.
Chemico-biological interactions, 230, 9-20 (2015-02-07)
The objective of the study was to evaluate the metabolism dependent inhibition of CYP2B6 catalyzed bupropion hydroxylation in human liver microsomes by monoamine oxidase (MAO) inhibitors and to predict the drug-drug interaction potential of monoamine oxidase inhibitors as perpetrators of
Yuki Ogawa et al.
European journal of pediatrics, 174(4), 509-518 (2014-09-25)
This study aimed to determine the population pharmacokinetics of doxapram in low-birth-weight (LBW) infants. A total of 92 serum concentration measurements that were obtained from 34 Japanese neonates were analyzed using nonlinear mixed-effect modeling (NONMEM). Estimates generated by NONMEM indicated
Christine R Klaus et al.
The Journal of pharmacology and experimental therapeutics, 350(3), 646-656 (2014-07-06)
EPZ-5676 [(2R,3R,4S,5R)-2-(6-amino-9H-purin-9-yl)-5-((((1r,3S)-3-(2-(5-(tert-butyl)-1H-benzo[d]imidazol-2-yl)ethyl)cyclobutyl)(isopropyl)amino)methyl)tetrahydrofuran-3,4-diol], a small-molecule inhibitor of the protein methyltransferase DOT1L, is currently under clinical investigation for acute leukemias bearing MLL-rearrangements (MLL-r). In this study, we evaluated EPZ-5676 in combination with standard of care (SOC) agents for acute leukemias as well
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.