L2807
3,7-Dimethyl-1,6-octadien-3-yl acetate
97%
Sinonimo/i:
Linalyl acetate, 3,7-Dimethyl-1,6-octadien-3-yl acetate, Bergamol
About This Item
Prodotti consigliati
Densità del vapore
6.8 (vs air)
Livello qualitativo
Tensione di vapore
0.1 mmHg ( 20 °C)
Saggio
97%
Indice di rifrazione
n20/D 1.453 (lit.)
P. ebollizione
220 °C (lit.)
Densità
0.901 g/mL at 25 °C (lit.)
Stringa SMILE
C\C(C)=C\CCC(C)(OC(C)=O)C=C
InChI
1S/C12H20O2/c1-6-12(5,14-11(4)13)9-7-8-10(2)3/h6,8H,1,7,9H2,2-5H3
UWKAYLJWKGQEPM-UHFFFAOYSA-N
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Categorie correlate
Avvertenze
Warning
Indicazioni di pericolo
Consigli di prudenza
Classi di pericolo
Eye Irrit. 2 - Skin Irrit. 2 - Skin Sens. 1B
Codice della classe di stoccaggio
10 - Combustible liquids
Classe di pericolosità dell'acqua (WGK)
WGK 1
Punto d’infiammabilità (°F)
201.2 °F - closed cup
Punto d’infiammabilità (°C)
94 °C - closed cup
Dispositivi di protezione individuale
Faceshields, Gloves, Goggles, type ABEK (EN14387) respirator filter
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
Protocolli
-Cymene; 2,5-Dimethylpyrrole; Acetoin, ≥96%, FCC, FG; 2,5-Dimethylpyrazine; 2,6-Dimethylpyrazine; 2-Ethylpyrazine, ≥98%, FG; 2,3-Dimethylpyrazine; 4-Heptanone; 3-Ethylpyridine; 2,3,5-Trimethylpyrazine; Furfural; Pyrrole; Furfuryl acetate; Linalool; Linalyl acetate; 5-Methylfurfural; γ-Butyrolactone; 2-Acetyl-1-methylpyrrole; Furfuryl alcohol; 2-Acetylpyrrole; Pyrrole-2-carboxaldehyde
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.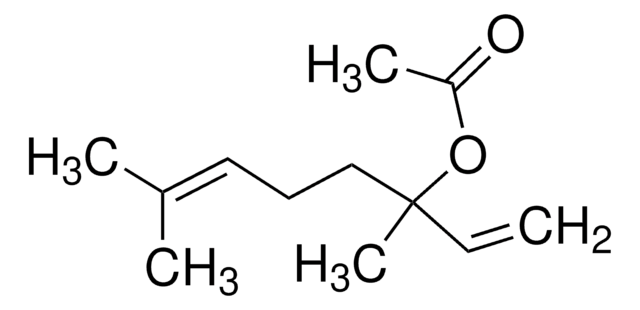




![Poly[2-methoxy-5-(2-ethylhexyloxy)-1,4-phenylenevinylene] average Mn 40,000-70,000](/deepweb/assets/sigmaaldrich/product/structures/344/488/b8f8179d-3970-4deb-a754-adda88cdb36f/640/b8f8179d-3970-4deb-a754-adda88cdb36f.png)
