B82200
4-Bromotoluene
98%
Sinonimo/i:
1-Methyl-4-bromobenzene, 4-Bromo-1-methylbenzene, 4-Methyl-1-bromobenzene, 4-Methylbromobenzene, 4-Tolyl bromide, p-Bromo(methyl)benzene, p-Methylbromobenzene, p-Tolyl bromide
About This Item
Prodotti consigliati
Livello qualitativo
Saggio
98%
Stato
solid
P. ebollizione
184 °C (lit.)
Punto di fusione
26-29 °C (lit.)
Densità
1.39 g/mL at 25 °C (lit.)
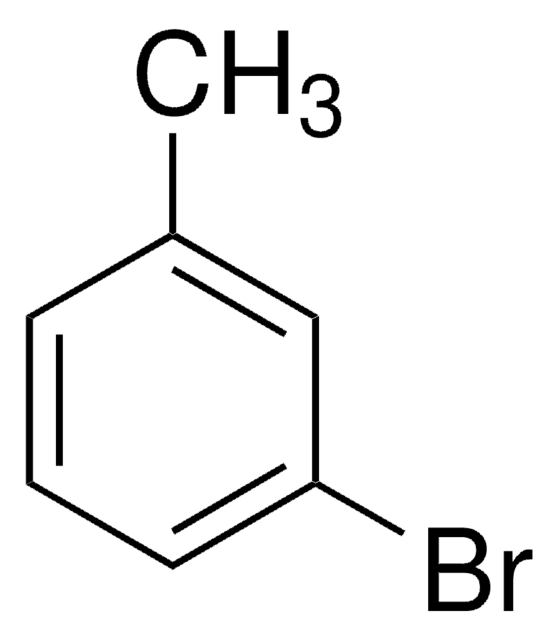
Stringa SMILE
Cc1ccc(Br)cc1
InChI
1S/C7H7Br/c1-6-2-4-7(8)5-3-6/h2-5H,1H3
ZBTMRBYMKUEVEU-UHFFFAOYSA-N
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Descrizione generale
Applicazioni
Avvertenze
Warning
Indicazioni di pericolo
Consigli di prudenza
Classi di pericolo
Acute Tox. 4 Inhalation - Aquatic Chronic 2
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Dispositivi di protezione individuale
dust mask type N95 (US), Eyeshields, Gloves
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Articoli
The Heck reaction is the palladium catalyzed cross-coupling reaction between alkenes and aryl or vinyl halides (or triflates) to afford substituted alkenes.
Global Trade Item Number
| SKU | GTIN |
|---|---|
| B82200-100G | 4061833442609 |
| B82200-500G | 4061838352743 |
| B82200-5G | 4061833442616 |
| B82200-5KG |
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.



![3-Bromo[1,1′-biphenyl]-4-ol AldrichCPR](/deepweb/assets/sigmaaldrich/product/structures/389/587/295f976f-c08c-41b9-94c0-64bfbe2cb6c1/640/295f976f-c08c-41b9-94c0-64bfbe2cb6c1.png)