A50606
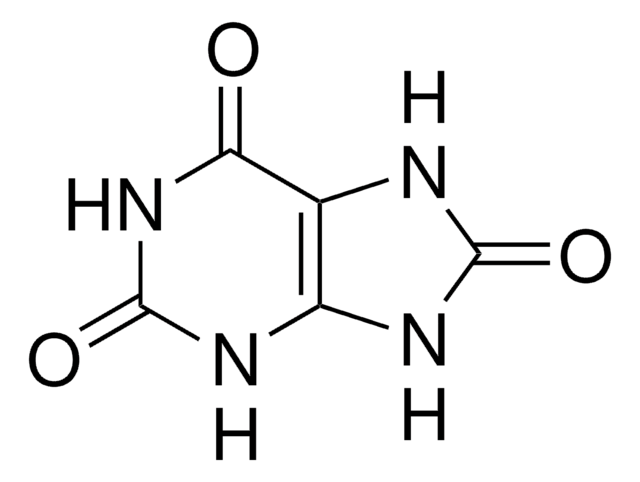
6-Aminouracil
97%
Sinonimo/i:
4-Amino-2,6-dihydroxypyrimidine, 6-Amino-2,4-pyrimidinediol
Autenticatiper visualizzare i prezzi riservati alla tua organizzazione & contrattuali
About This Item
Formula empirica (notazione di Hill):
C4H5N3O2
Numero CAS:
Peso molecolare:
127.10
Beilstein:
120491
Numero CE:
Numero MDL:
Codice UNSPSC:
12352100
ID PubChem:
NACRES:
NA.22
Prodotti consigliati
Livello qualitativo
Saggio
97%
Forma fisica
powder
Punto di fusione
≥360 °C (lit.)
Stringa SMILE
Nc1cc(O)nc(O)n1
InChI
1S/C4H5N3O2/c5-2-1-3(8)7-4(9)6-2/h1H,(H4,5,6,7,8,9)
LNDZXOWGUAIUBG-UHFFFAOYSA-N
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Dispositivi di protezione individuale
Eyeshields, Gloves, type N95 (US)
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
H Sladowska et al.
Acta poloniae pharmaceutica, 53(1), 39-46 (1996-01-01)
Synthesis of 6-substituted 2,4-dioxo-1,2,3,4,5,6,7,8-octahydropyrimido[4,5-d]pyrimidines [III-VI] obtained by cyclocondensation of 1-phenyl-6-aminouracil with formaline and the primary amines is described. Compounds III, V, VI in the Mannich reaction with secondary cyclic amines yield the corresponding 3-substituted N-aminomethyl derivatives VII-X. Some of them
Kyung Mee Kim et al.
Journal of chromatography. B, Analytical technologies in the biomedical and life sciences, 877(1-2), 65-70 (2008-12-17)
Uric acid (UA) can be directly converted to allantoin enzymatically by uricase in most mammals except humans or by reaction with superoxide. UA can react directly with nitric oxide to generate 6-aminouracil and with peroxynitrite to yield triuret; both of
María-Jesús Pérez-Pérez et al.
Mini reviews in medicinal chemistry, 5(12), 1113-1123 (2005-12-27)
Thymidine Phosphorylase (TPase) catalyses the reversible phosphorolysis of pyrimidine 2'-deoxynucleosides to 2-deoxyribose-1-phosphate and their respective pyrimidine bases, including the phosphorolysis of nucleoside analogues with important antiviral or anticancer properties. Moreover, TPase, identified also as the angiogenic platelet-derived endothelial cell growth
Christine Gersch et al.
Nucleosides, nucleotides & nucleic acids, 27(8), 967-978 (2008-08-13)
The 1980 identification of nitric oxide (NO) as an endothelial cell-derived relaxing factor resulted in an unprecedented biomedical research of NO and established NO as one of the most important cardiovascular, nervous and immune system regulatory molecule. A reduction in
K Hirota et al.
Nucleic acids symposium series, (37)(37), 59-60 (1997-01-01)
Inhibitors of thymidine phosphorylase (dThdPase) are expected to suppress the growth and metastasis of tumor cells by inhibition of angiogenesis and were designed by utilizing the three dimensional structure of the enzyme. 5-Substituted 6-aminouracils (5) and 7-substituted pyrrolo[2,3-d]pyrimidine-2,4-diones (6) were
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.