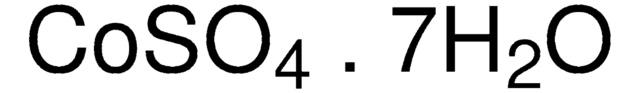939331
Nickel(II) sulfate hexahydrate

≥99.9% trace metals basis
Sinonimo/i:
Nickelous Sulfate, Hexahydrate, Battery grade nickel sulfate, Nickel monosulfate hexahydrate, Nickel sulphate hexahydrate
About This Item
Prodotti consigliati
Tipo
(High purity Salts)
Livello qualitativo
Saggio
≥99.9% trace metals basis
98-102% (EDTA, complexometric)
Stato
powder or crystals
Impurezze
≤1000 ppm trace metals basis
Colore
faint blue to dark blue-green
pH
4.3-4.7 (20 °C, 100 g/L in water)
Solubilità
water: soluble
Densità
2.07 g/cm3 at 20 °C
Anioni in tracce
chloride (Cl-): ≤20 ppm
Cationi in tracce
Al: <50 ppm
Ca: <50 ppm
Co: <50 ppm
Cu: <50 ppm
Fe: <50 ppm
K: <50 ppm
Mg: <50 ppm
Na: <50 ppm
Pb: <50 ppm
Zn: <50 ppm
Stringa SMILE
[Ni+2].[S](=O)(=O)([O-])[O-].O.O.O.O.O.O
InChI
1S/Ni.H2O4S.6H2O/c;1-5(2,3)4;;;;;;/h;(H2,1,2,3,4);6*1H2/q+2;;;;;;;/p-2
RRIWRJBSCGCBID-UHFFFAOYSA-L
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Descrizione generale
Applicazioni
Therefore, Nickel(II) sulfate hexahydrate has been widely used as a key component for the synthesis of Cathode for Lithium-ion batteries. - Spherical NCM 622 and Li[Ni0.9Co0.05Mn0.05]O2 (NCM 900505) were synthesized via a co-precipitation method using Nickel(II) sulfate hexahydrate. In order to achieve the desired energy density, it is necessary to maximize both the nickel content in the cathode and the cutoff voltage. [] - to synthesis single-crystal, Ni-rich NCM and polycrystalline NCM cathodes with various Ni content by coprecipitation method . These Ni-rich layered cathodes like NCM, NCA, and NCMA ([Ni1–x–yCox(Mn and/or Al)y]O2) are the top choices for powering upcoming electric vehicles. It is found that polycrystalline NCM cathodes are prone to intergranular microcracking during cycling, single crystal NCM cathodes demonstrate resilience against mechanical fracture, even under highly charged conditions or repeated cycles. Due to limited lithium-ion diffusion pathways, the electrochemical performance of single crystal -NCM cathodes, particularly in terms of capacity and cycling stability, is lower compared to that of polycrystalline-NCM cathodes. The difference in the electrochemical performance of single crystal -NCM and polycrystalline-NCM cathodes grows as the Ni fraction increases. In addition, Nickel(II) sulfate hexahydrate is widely used for electroplating for producing metallic coatings. Nickel(II) sulfate hexahydrate can also be used as a catalyst in: -Pt50Ni50 catalysts supported on MCM-41 were produced using wet co-impregnation. These catalysts were then employed for hydrogenation reactions of benzene in gas phase. The morphology of the metal phase within the catalysts has a notable impact on the conversion of benzene to cyclohexane. Factors like reduction temperature, NaBH4 concentration, and reduction medium influence the particle morphology. []
Caratteristiche e vantaggi
- Water soluble
- Medium purity (99.9%)
- Low trace metals in ppm level
- Cost effective Suitable for battery applications
- Recycled catalyst
Avvertenze
Danger
Indicazioni di pericolo
Consigli di prudenza
Classi di pericolo
Acute Tox. 4 Inhalation - Acute Tox. 4 Oral - Aquatic Acute 1 - Aquatic Chronic 1 - Carc. 1A Inhalation - Muta. 2 - Repr. 1B - Resp. Sens. 1 - Skin Irrit. 2 - Skin Sens. 1 - STOT RE 1 Inhalation
Organi bersaglio
Respiratory Tract
Codice della classe di stoccaggio
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Scegli una delle versioni più recenti:
Certificati d'analisi (COA)
It looks like we've run into a problem, but you can still download Certificates of Analysis from our Documenti section.
Se ti serve aiuto, non esitare a contattarci Servizio Clienti
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.