550426
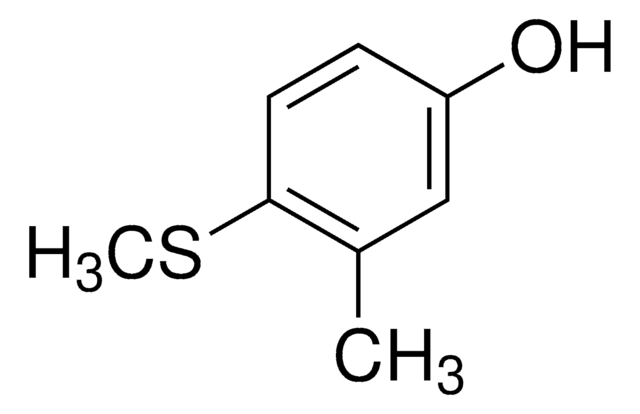
4-(Methylmercapto)phenol
98%
Sinonimo/i:
4-Hydroxythioanisole
Autenticatiper visualizzare i prezzi riservati alla tua organizzazione & contrattuali
About This Item
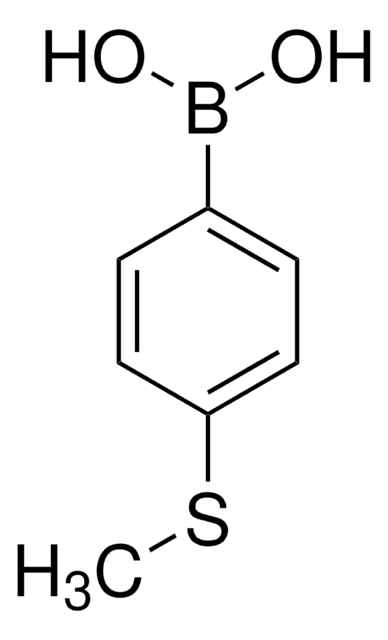
Formula condensata:
CH3SC6H4OH
Numero CAS:
Peso molecolare:
140.20
Numero CE:
Numero MDL:
Codice UNSPSC:
12352100
ID PubChem:
NACRES:
NA.22
Prodotti consigliati
Livello qualitativo
Saggio
98%
P. ebollizione
153-156 °C/20 mmHg (lit.)
Punto di fusione
84-86 (lit.)
Stringa SMILE
CSc1ccc(O)cc1
InChI
1S/C7H8OS/c1-9-7-4-2-6(8)3-5-7/h2-5,8H,1H3
QASBCTGZKABPKX-UHFFFAOYSA-N
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Categorie correlate
Descrizione generale
4-(Methylmercapto)phenol (4MP) mediates the removal of benzyloxycarbonyl and O-benzyl protecting groups by accepting the benzyl groups during the acidolytic cleavage with trifluoroacetic acid. The presence of hydroxyl group in the para position enhances the rate of hydrodesulfurization (HDS) of 4MP.
Applicazioni
4-(Methylmercapto)phenol [4-(Methylthio)phenol] may be used in the preparation of phosphoramidodithioate intermediates for the synthesis of sulprofos amidate.
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Dispositivi di protezione individuale
dust mask type N95 (US), Eyeshields, Gloves
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
Lianming Wu et al.
Journal of mass spectrometry : JMS, 44(9), 1389-1394 (2009-08-22)
A novel ion/molecule reaction was observed to occur under electrospray ionization (ESI), atmospheric pressure chemical ionization (APCI), and atmospheric pressure photo ionization (APPI) conditions, leading to dimerization of ionized 4-(methyl mercapto)-phenol followed by fast H(*) loss. The reaction is particularly
Acceptors in the removal of protecting groups.
Bodanszky M and Bodanszky A.
International Journal of Peptide and Protein Research, 23(3), 287-291 (1984)
Hydrotreating of compounds containing both oxygen and sulfur: effect of para-hydroxyl substituent on the reactions of mercapto and methylmercapto groups.
Viljava TR and Krause AOI.
Applied Catalysis A: General, 145(1), 237-251 (1996)
Resolution and stereoselective action of sulprofos and related S-propyl phosphorothiolates.
Hirashima A, et al.
Journal of Agricultural and Food Chemistry, 32(6), 1302-1307 (1984)
Hua Zhang et al.
Molecules (Basel, Switzerland), 15(1), 83-92 (2010-01-30)
A highly efficient transition-metal-free catalytic system Br2/NaNO2/H2O has been developed for a robust and economic acid-free aerobic oxidation of sulfides. It is noteworthy that the sulfide function reacts under mild conditions without over-oxidation to sulfone. The role of NaNO2as an
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.