433829
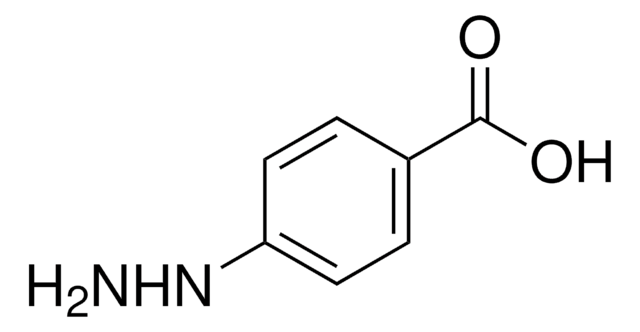
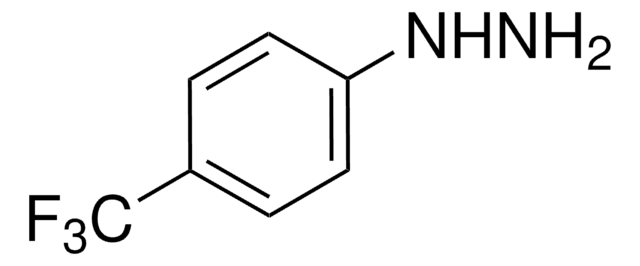
4-Hydrazinobenzoic acid hydrochloride
98%
Autenticatiper visualizzare i prezzi riservati alla tua organizzazione & contrattuali
About This Item
Formula condensata:
H2NNHC6H4CO2H · HCl
Numero CAS:
Peso molecolare:
188.61
Numero CE:
Numero MDL:
Codice UNSPSC:
12352100
ID PubChem:
NACRES:
NA.22
Prodotti consigliati
Livello qualitativo
Saggio
98%
Punto di fusione
253 °C (dec.) (lit.)
Gruppo funzionale
carboxylic acid
hydrazine
Temperatura di conservazione
2-8°C
Stringa SMILE
Cl.NNc1ccc(cc1)C(O)=O
InChI
1S/C7H8N2O2.ClH/c8-9-6-3-1-5(2-4-6)7(10)11;/h1-4,9H,8H2,(H,10,11);1H
XHLQMKQBCHYRLC-UHFFFAOYSA-N
Categorie correlate
Descrizione generale
4-Hydrazinobenzoic acid (p-Hydrazinobenzoic acid, HBA) has been reported as an ingredient of the cultivated mushroom Agaricus bisporus. It is carcinogenic to rodents. Mechanism of DNA damage by 4-hydrazinobenzoic acid has been studied by using 32P-labeled DNA fragments obtained from the human p53 and p16 tumor suppressor genes. It may be used for the preparation of 4-(3-(2-(tert-butoxycarbonylamino)ethyl)-5-hydroxy-1H-pyrazol-1-yl)benzoic acid.
Avvertenze
Warning
Indicazioni di pericolo
Consigli di prudenza
Classi di pericolo
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Organi bersaglio
Respiratory system
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Dispositivi di protezione individuale
dust mask type N95 (US), Eyeshields, Gloves
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
Synthesis of 3-(2-aminoethyl)-5-hydroxy-1H-pyrazole derivatives.
Groselj U, et al.
ARKIVOC (Gainesville, FL, United States), 3, 49-65 (2012)
B M McManus et al.
Laboratory investigation; a journal of technical methods and pathology, 57(1), 78-85 (1987-07-01)
p-Hydrazinobenzoic acid (HBA), an ingredient of the cultivated mushroom Agaricus bisporus, was given in hydrochloride form at a dosage of 0.125% in drinking water for life to randomly bred Swiss mice. Previous studies had demonstrated that either synthetic or naturally
Yun-Gon Kim et al.
Chemistry & biology, 15(3), 215-223 (2008-03-22)
Glycan recognition leading to cell-cell interactions, signaling, and immune responses is mediated by various glycan-binding proteins (GBPs) showing highly diverse ligand specificities. We describe here a rapid glycan immobilization technique via 4-hydrazinobenzoic acid (HBA)-functionalized beads and its application to high-throughput
Yan-Juan Gu et al.
International journal of nanomedicine, 6, 2889-2898 (2011-12-02)
Single-walled carbon nanotubes (SWNTs) have been identified as an efficient drug carrier. Here a controlled drug-delivery system based on SWNTs coated with doxorubicin (DOX) through hydrazone bonds was developed, because the hydrazone bond is more sensitive to tumor microenvironments than
X Wang et al.
Carbohydrate research, 332(2), 191-196 (2001-07-04)
p-Hydrazinobenzenesulfonic acid was explored as an ultraviolet labeling reagent for capillary electrophoresis of mono-, di- and trisaccharides. The labeling reaction that produces p-sulfophenylhydrazines took less than 8 min, and introduced both chromphore and charged groups into the carbohydrate molecules. The
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.