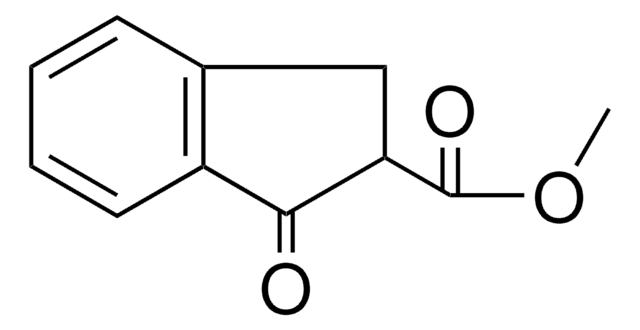
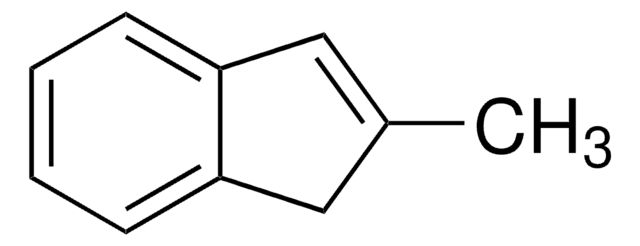
391743
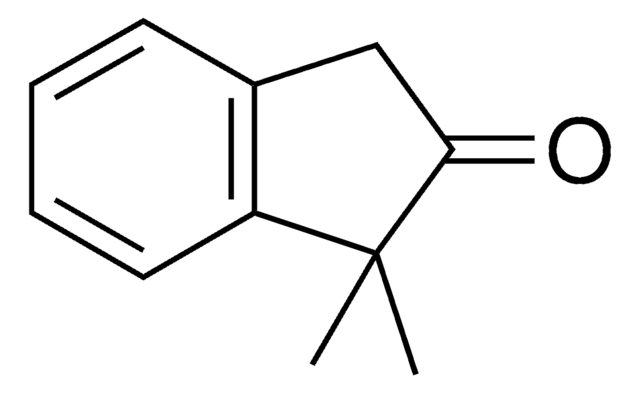
2-Methyl-1-indanone
99%
Autenticatiper visualizzare i prezzi riservati alla tua organizzazione & contrattuali
About This Item
Formula empirica (notazione di Hill):
C10H10O
Numero CAS:
Peso molecolare:
146.19
Numero MDL:
Codice UNSPSC:
12352100
ID PubChem:
NACRES:
NA.22
Prodotti consigliati
Livello qualitativo
Saggio
99%
Stato
liquid
Indice di rifrazione
n20/D 1.555 (lit.)
P. ebollizione
93-95 °C/4 mmHg (lit.)
Densità
1.064 g/mL at 25 °C (lit.)
Gruppo funzionale
ketone
Stringa SMILE
CC1Cc2ccccc2C1=O
InChI
1S/C10H10O/c1-7-6-8-4-2-3-5-9(8)10(7)11/h2-5,7H,6H2,1H3
BEKNOGMQVKBMQN-UHFFFAOYSA-N
Categorie correlate
Descrizione generale
2-Methyl-1-indanone, a α-benzocycloalkenone, is a derivative of 1-indanone. Its synthesis has been reported. The enzymatic dynamic kinetic resolution (DKR) of racemic 2-methyl-1-indanone has been studied. The asymmetric α-arylation and hydroxymethylation of 2-methyl-1-indanone has been reported. It participated in the synthesis of 2-methyl-6-carboxyazulene.
Applicazioni
2-Methyl-1-indanone may be used as a starting material in the synthesis of β-benzocycloalkenone. It may be used in the synthesis of the following:
- cyclohex-2-en-1-yl 2-methyl-1H-inden-3-yl carbonate
- 2-hydroxy-2-methyl-1-indanone
- O-alkoxycarbonylation of lithium enolates
Codice della classe di stoccaggio
10 - Combustible liquids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Dispositivi di protezione individuale
Eyeshields, Gloves
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
Michael W Justik et al.
Molecules (Basel, Switzerland), 10(1), 217-225 (2007-11-17)
The conversion of alpha-benzocycloalkenones to homologous beta-benzocyclo-alkenones containing six, seven and eight-membered rings is reported. This was accomplished via a Wittig olefination-oxidative rearrangement sequence using[hydroxy(tosyloxy)iodo]-benzene (HTIB) is the oxidant, that enables the synthesis of regioisomeric pairs of methyl-substituted beta-benzocycloalkenones. The
Cyclophanes. 9. anti-[2.2](2, 6) Azulenophane. Synthesis and charge-transfer interaction.
Luhowy R and Keehn PM.
Journal of the American Chemical Society, 99(11), 3797-3805 (1977)
On the decarboxylation of 2-methyl-1-tetralone-2-carboxylic acid-oxidation of the enol intermediate by triplet oxygen.
Riahi A, et al.
New. J. Chem., 37(8), 2245-2249 (2013)
Taku Kitanosono et al.
Chemistry, an Asian journal, 10(1), 133-138 (2014-10-29)
Enzymes exhibit overwhelmingly superior catalysis compared with artificial catalysts. Current strategies to rival enzymatic catalysis require unmodified or minimally modified structures of active sites, gigantic molecular weight, and sometimes the use of harsh conditions such as extremely low temperatures in
Selective and easy preparation of enol carbonates of α-disubstituted aryl ketones from their lithium enolates.
Aboulhoda SJ, et al.
Tetrahedron Letters, 36(27), 4795-4796 (1995)
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.