343382
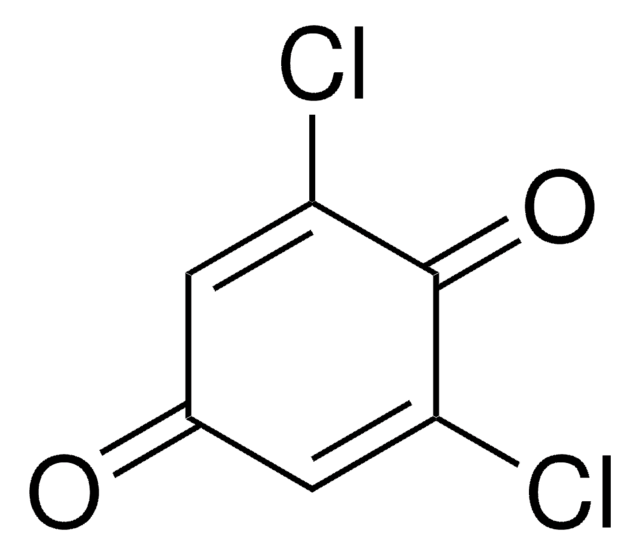
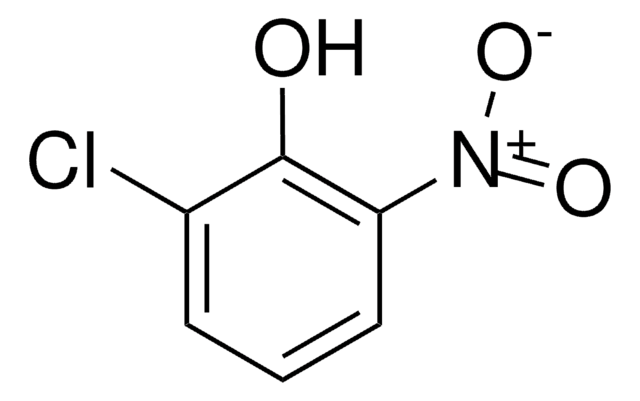
2-Chloro-1,4-benzoquinone
95%
Autenticatiper visualizzare i prezzi riservati alla tua organizzazione & contrattuali
About This Item
Formula empirica (notazione di Hill):
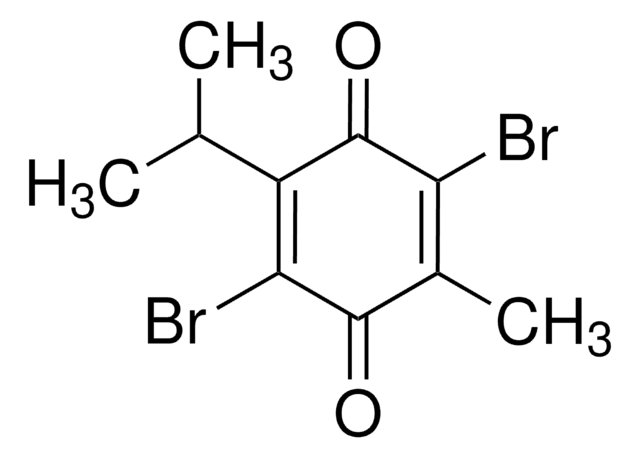
C6H3ClO2
Numero CAS:
Peso molecolare:
142.54
Numero MDL:
Codice UNSPSC:
12352100
ID PubChem:
NACRES:
NA.22
Prodotti consigliati
Livello qualitativo
Saggio
95%
Gruppo funzionale
chloro
ketone
Stringa SMILE
ClC1=CC(=O)C=CC1=O
InChI
1S/C6H3ClO2/c7-5-3-4(8)1-2-6(5)9/h1-3H
WOGWYSWDBYCVDY-UHFFFAOYSA-N
Categorie correlate
Descrizione generale
2-Chloro-1,4-benzoquinone is a quinone derivative. It is one of the intermediate formed during the degradation of 3,4-dichloroaniline in a dielectric barrier discharge plasma reactor. It is formed during lignin peroxidase catalyzed oxidation of 2-chloro-1,4-dimethoxybenzene. 2-Chloro-1,4-benzoquinone on dechlorination yields 1,2,4-trihydroxybenzene.
Applicazioni
2-Chloro-1,4-benzoquinone may be used in the preparation of chloro derivatives of prenylnaphthohydroquinone.
Avvertenze
Warning
Indicazioni di pericolo
Consigli di prudenza
Classi di pericolo
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Organi bersaglio
Respiratory system
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Dispositivi di protezione individuale
dust mask type N95 (US), Eyeshields, Gloves
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Aurora Molinari et al.
Bioorganic & medicinal chemistry, 13(11), 3841-3846 (2005-06-21)
From the Diels-Alder adduct between alpha-myrcene and 2-chloro-1,4-benzoquinone, a family of chloro derivatives of prenylnaphthohydroquinone have been synthesised and evaluated for their cytotoxicity against 14 neoplastic cell lines.
Emily N Vebrosky et al.
Journal of agricultural and food chemistry, 67(27), 7609-7615 (2019-07-02)
Shallow water systems are uniquely susceptible to environmental processes such as photolysis and hydrolysis that can influence the dissipation of pesticides into sediments. The fungicide dicloran has previously been shown to undergo photolysis and is reported to dissipate in soils
An ecologically effective water treatment technique using electrochemically generated hydroxyl radicals for in situ destruction of organic pollutants: Application to herbicide 2, 4-D.
Oturan MA.
J. Appl. Electrochem., 30(4), 475-482 (2000)
R Grey et al.
Journal of basic microbiology, 38(5-6), 371-382 (1999-01-01)
The white-rot fungus Trametes versicolor was used to study the influence of extracellular laccase activity on the degradation of 2-chlorophenol (2-CP) and the formation of metabolites under conditions, characterized by the absence of other phenol-oxidizing enzymes. 2-CP enhanced the production
P J Teunissen et al.
Archives of biochemistry and biophysics, 360(2), 233-238 (1998-12-16)
2-Chloro-1,4-dimethoxybenzene (2Cl-1,4-DMB) oxidation by lignin peroxidase (LiP) proceeds via the formation of the 2Cl-1,4-DMB cation radical as indicated by ESR and UV/vis spectroscopy. The products of the LiP-catalyzed oxidation of 2Cl-1,4-DMB were identified as 2-chloro-1,4-benzoquinone and the dimers dichlorotetramethoxybiphenyl and
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.