327972
Aceanthrenequinone
96%
Sinonimo/i:
1,2-Aceanthrylenedione
Autenticatiper visualizzare i prezzi riservati alla tua organizzazione & contrattuali
About This Item
Formula empirica (notazione di Hill):
C16H8O2
Numero CAS:
Peso molecolare:
232.23
Numero MDL:
Codice UNSPSC:
12352100
ID PubChem:
NACRES:
NA.22
Prodotti consigliati
Saggio
96%
Forma fisica
solid
Punto di fusione
270-273 °C (lit.)
Gruppo funzionale
ketone
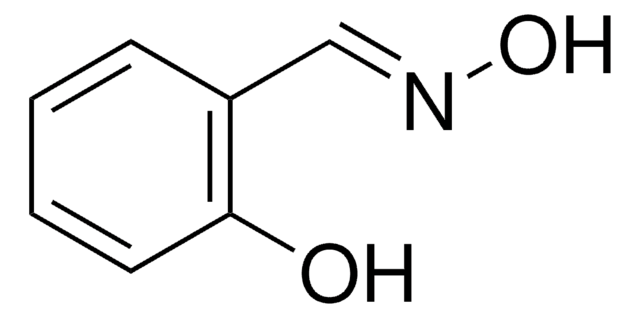
Stringa SMILE
O=C1C(=O)c2c3ccccc3cc4cccc1c24
InChI
1S/C16H8O2/c17-15-12-7-3-5-10-8-9-4-1-2-6-11(9)14(13(10)12)16(15)18/h1-8H
YAIBDWAANBTYIA-UHFFFAOYSA-N
Descrizione generale
Aceanthrenequinone is a cyclic α-diketone. It reacts with hexaethyltriaminophosphine in the presence of fullerene C(60), to yield methanofullerene derivatives. Hydroxyalkylation reactions of aceanthrenequinone with a series of arenes was reported.
Applicazioni
Aceanthrenequinone was used in synthesis of spiro-tricyclic porphodimethenes.
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Dispositivi di protezione individuale
Eyeshields, Gloves, type N95 (US)
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
M Harmjanz et al.
Organic letters, 3(15), 2281-2284 (2001-07-21)
[structure: see text] Acid-catalyzed [2 + 2] condensation reactions of polycyclic aromatic vicinal diketones including aceanthrenequinone, phenathrenequinone, and pyrene-4,5-dione with 5-mesityldipyrromethanes are outlined, and this methodology provides a flexible entry to spiro-tricyclic porphodimethenes. The porphodimethene products have been fully characterized
Douglas A Klumpp et al.
Applied catalysis. A, General, 336(1-2), 128-132 (2008-03-01)
The hydroxyalkylation reactions of aceanthrenequinone (6) and acenapthenequinone (7) with a series of arenes have been studied. In reactions with the Brønsted superacid CF(3)SO(3)H (triflic acid), the condensation products are formed in good yields (58-99%, 10 examples) with high regioselectivity.
Irina P Romanova et al.
The Journal of organic chemistry, 76(8), 2548-2557 (2011-03-12)
The reactions of such cyclic α-diketones as acenaphthenequinone, aceanthrenequinone, and N-alkylisatins, with hexaethyltriaminophosphine in the presence of the fullerene C(60), lead to the formation of methanofullerene derivatives under mild conditions. This process proceeds via deoxygenation of the dicarbonyl compound by
Janice L Hyatt et al.
Journal of medicinal chemistry, 50(23), 5727-5734 (2007-10-19)
Carboxylesterases (CE) are ubiquitous enzymes responsible for the detoxification of xenobiotics, including numerous clinically used drugs. Therefore, the selective inhibition of these proteins may prove useful in modulating drug half-life and bioavailability. Recently, we identified 1,2-diones as potent inhibitors of
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.