301868
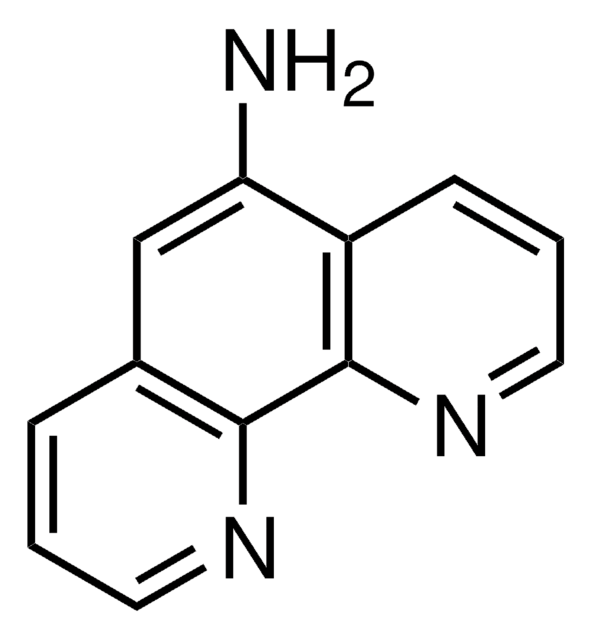
4,7-Phenanthroline
98%
Autenticatiper visualizzare i prezzi riservati alla tua organizzazione & contrattuali
About This Item
Formula empirica (notazione di Hill):
C12H8N2
Numero CAS:
Peso molecolare:
180.21
Numero MDL:
Codice UNSPSC:
12352100
ID PubChem:
NACRES:
NA.22
Prodotti consigliati
Livello qualitativo
Saggio
98%
Punto di fusione
172-174 °C (lit.)
Stringa SMILE
c1cnc2ccc3ncccc3c2c1
InChI
1S/C12H8N2/c1-3-9-10-4-2-8-14-12(10)6-5-11(9)13-7-1/h1-8H
DATYUTWESAKQQM-UHFFFAOYSA-N
Categorie correlate
Descrizione generale
4,7-Phenanthroline reacts with ruthenium carbonyl to yield cyclometalated derivatives.
Applicazioni
4,7-Phenanthroline was used in preparation of:
- cyclic tetranuclear half-sandwich ruthenium(II) complexes
- positively charged homochiral cyclic trinuclear metallacalix[3]arene species
Avvertenze
Danger
Indicazioni di pericolo
Consigli di prudenza
Classi di pericolo
Acute Tox. 4 Oral - Eye Dam. 1
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Dispositivi di protezione individuale
Eyeshields, Gloves, type N95 (US)
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Julien Frey et al.
Journal of the American Chemical Society, 130(33), 11013-11022 (2008-07-26)
Variously substituted coordinating rigid rods have been synthesized which incorporate a central 4,7-phenanthroline nucleus attached to two 2-pyridyl groups via its 3 and 8 positions, so as to yield bis-bidentate chelates, the two-coordinating axes of the chelates being parallel to
Miguel A Galindo et al.
Journal of inorganic biochemistry, 102(5-6), 1025-1032 (2008-01-12)
The reaction between [(eta(6)-p-cymene)Ru(H2O)3]X2 and 4,7-phenanthroline (phen) leads to the formation of the rectangular tetranuclear complexes [(eta(6)-p-cymene)4Ru4(mu-4,7-phen-N4,N7)(2)(mu-OH)4]X4 (X=NO3, 1a; SO3CF3, 1b) which have been structurally characterised by X-ray crystallography. 1H NMR spectroscopic studies suggest the presence of a partially dissociated
Miguel A Galindo et al.
Dalton transactions (Cambridge, England : 2003), (10)(10), 1563-1566 (2004-07-15)
Reaction of [(dach)Pd(NO3)2] entities (dach = (R,R)-1,2-diaminocyclohexane, (S,S)-1,2-diaminocyclohexane) and 4,7-phenanthroline (phen) providing, respectively, 90 and 120 degrees bond angles, leads to the formation of two novel positively charged homochiral cyclic trinuclear metallacalix[3]arene species [((R,R)-1,2-diaminocyclohexane)Pd(phen)]3(NO3)6 (2a) and [((S,S)-1,2-diaminocyclohexane)Pd(phen)]3(NO3)6 (2b). These species
L W Mitchell et al.
Archives of biochemistry and biophysics, 300(1), 169-177 (1993-01-01)
Porphobilinogen synthase (PBGS) is essential to all life forms; in mammals it is definitively established that Zn(II) is required for activity. The literature regarding the metal requirement for PBGS in other animals, plants, and bacteria neither establishes nor disproves a
V Arluison et al.
Biochemistry, 37(20), 7268-7276 (1998-06-04)
RNA:pseudouridine synthetase (Pus1) from Saccharomyces cerevisiae is a multisite specific enzyme that catalyzes the formation of pseudouridine at positions 34 and 36 of intron-containing precursor tRNAIle and at positions 27 and/or 28 of several yeast tRNAs. In this paper we
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.

![Pyrazino[2,3-f][1,10]phenanthroline 99% (HPLC)](/deepweb/assets/sigmaaldrich/product/structures/226/341/31d3909e-6700-4a3e-bfb3-9ed1f6b66ee2/640/31d3909e-6700-4a3e-bfb3-9ed1f6b66ee2.png)