275352
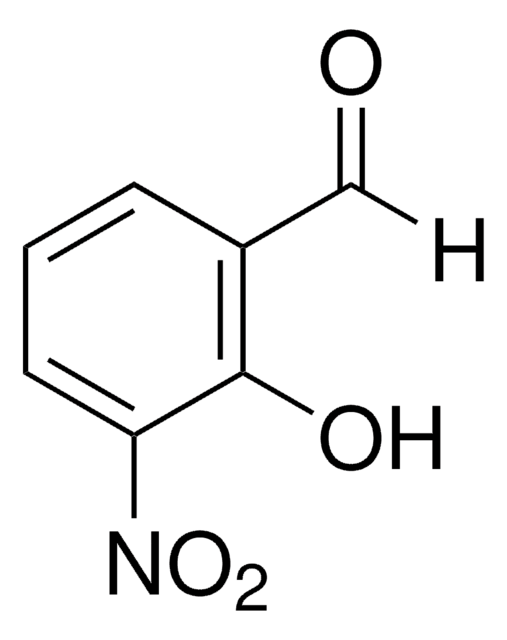
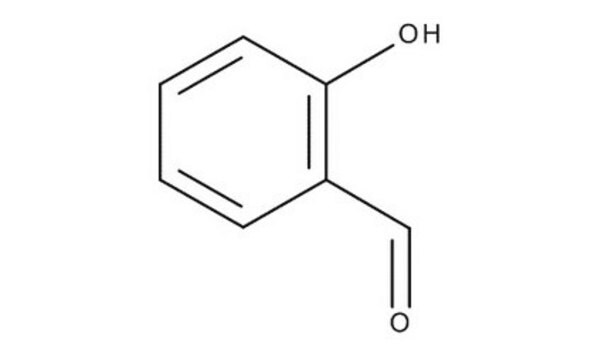
2-Hydroxy-5-nitrobenzaldehyde
98%
Sinonimo/i:
5-Nitrosalicylaldehyde
Autenticatiper visualizzare i prezzi riservati alla tua organizzazione & contrattuali
About This Item
Formula condensata:
HOC6H3(NO2)CHO
Numero CAS:
Peso molecolare:
167.12
Beilstein:
512565
Numero CE:
Numero MDL:
Codice UNSPSC:
12352100
ID PubChem:
NACRES:
NA.22
Prodotti consigliati
Livello qualitativo
Saggio
98%
Punto di fusione
125-128 °C (lit.)
Gruppo funzionale
aldehyde
nitro
Stringa SMILE
[H]C(=O)c1cc(ccc1O)[N+]([O-])=O
InChI
1S/C7H5NO4/c9-4-5-3-6(8(11)12)1-2-7(5)10/h1-4,10H
IHFRMUGEILMHNU-UHFFFAOYSA-N
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Descrizione generale
2-Hydroxy-5-nitrobenzaldehyde is a nitroaromatic compound used to prepare Schiff base ligands.
The interaction of 2-hydroxy-5-nitrobenzaldehyde and chlorogenic acid (CHL) with the components of the rat hepatic glucose 6-phosphatase system was studied.
The interaction of 2-hydroxy-5-nitrobenzaldehyde and chlorogenic acid (CHL) with the components of the rat hepatic glucose 6-phosphatase system was studied.
Proprietà fisiche
Free of 3-nitro isomer
Avvertenze
Warning
Indicazioni di pericolo
Consigli di prudenza
Classi di pericolo
Acute Tox. 4 Oral - Eye Irrit. 2 - Skin Irrit. 2
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Dispositivi di protezione individuale
dust mask type N95 (US), Eyeshields, Gloves
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Anke Rüttger et al.
BioTechniques, 41(4), 469-473 (2006-10-31)
A method is described allowing the selective determination of four cathepsins (B, H, K, and L) in live cells. Adherently growing cells are incubated with partially selective substrates for each cathepsin (peptidic derivatives of 4-methoxy-beta-naphthylamine) in microtiter plates together with
W J Arion et al.
Archives of biochemistry and biophysics, 339(2), 315-322 (1997-03-15)
We have studied the interactions of chlorogenic acid (CHL) and 2-hydroxy-5-nitrobenzaldehyde (HNB) with the components of the rat hepatic glucose 6-phosphatase (Glc-6-Pase) system. CHL and HNB are competitive inhibitors of glucose 6-phosphate (Glc-6-P) hydrolysis in intact microsomes with Ki values
M A Pajares et al.
The Journal of biological chemistry, 264(12), 6804-6809 (1989-04-25)
The 11-cis-retinal binding site of rhodopsin is of great interest because it is buried in the membrane but yet must provide an environment for charged amino acids. In addition, the active-site lysine residue must be able to engage in rapid
Anda-Mihaela Olaru et al.
Carbohydrate polymers, 179, 59-70 (2017-11-08)
A series of hydrogels based on chitosan polyamine and nitrosalicylaldehyde were prepared via dynamic covalent chemistry (DCC), by imination and transimination reactions towards ordered clusters which play the role of crosslinking nodes of the chitosan network. The hydrogelation mechanism has
Anas G Elsafy et al.
Sensors (Basel, Switzerland), 18(7) (2018-07-13)
(E)-2-((benzo[d]thiazol-2-ylimino)methyl)-4-nitrophenol 1 and (E)-2-(((6-methoxybenzo[d]thiazol-2-yl)imino)methyl)-4-nitrophenol 2 were synthesized efficiently under microwave conditions. The structures were confirmed using IR, ¹H NMR, and 13C NMR. UV-vis. Fluorescence investigations demonstrated that 1 and 2 are sensitive and selective sensors for detection of cyanide over
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.