238082
4-Butoxybenzaldehyde
98%
Autenticatiper visualizzare i prezzi riservati alla tua organizzazione & contrattuali
About This Item
Formula condensata:
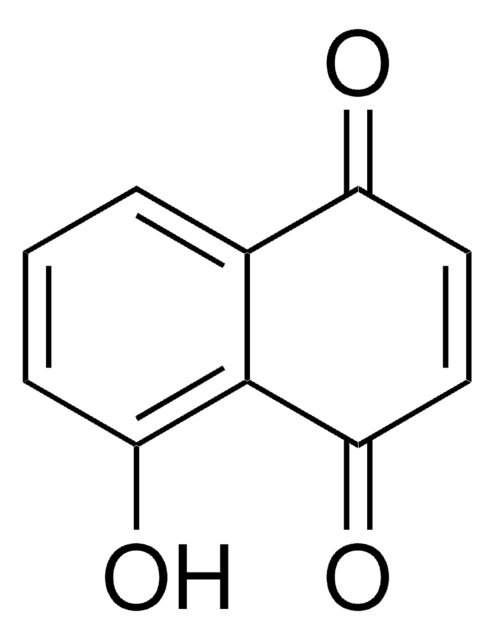
CH3(CH2)3OC6H4CHO
Numero CAS:
Peso molecolare:
178.23
Numero CE:
Numero MDL:
Codice UNSPSC:
12352100
ID PubChem:
NACRES:
NA.22
Prodotti consigliati
Livello qualitativo
Saggio
98%
Forma fisica
liquid
Indice di rifrazione
n20/D 1.539 (lit.)
P. eboll.
285 °C (lit.)
Densità
1.031 g/mL at 25 °C (lit.)
Gruppo funzionale
aldehyde
Stringa SMILE
CCCCOc1ccc(C=O)cc1
InChI
1S/C11H14O2/c1-2-3-8-13-11-6-4-10(9-12)5-7-11/h4-7,9H,2-3,8H2,1H3
XHWMNHADTZZHGI-UHFFFAOYSA-N
Categorie correlate
Descrizione generale
Kinetic constant for the inhibition of the diphenolase activity of mushroom tyrosinase by 4-butoxybenzaldehyde has been evaluated.
Applicazioni
4-Butoxybenzaldehyde has been used in the synthesis of:
- 6-amino-4-(4-butoxyphenyl)-3,5-dicyanopyridine-2(1H)-thione
- 16-(p-butoxybenzylidene)androsta-1,4-diene-3,17-dione via condensation reaction with androsta-1,4-diene-3,17-dione
Note legali
Darkens in storage with no loss in purity
Avvertenze
Warning
Indicazioni di pericolo
Consigli di prudenza
Classi di pericolo
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Organi bersaglio
Respiratory system
Codice della classe di stoccaggio
10 - Combustible liquids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
>235.4 °F - closed cup
Punto d’infiammabilità (°C)
> 113 °C - closed cup
Dispositivi di protezione individuale
Eyeshields, Gloves, type ABEK (EN14387) respirator filter
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
16-(p-Butoxybenzylidene) androsta-1, 4-diene-3, 17-dione.
Ogawa K, et al.
Acta Crystallographica Section C, Crystal Structure Communications, 48(7), 1359-1361 (1992)
Michael reaction in synthesis of 6-amino-4-(4-butoxyphenyl)-3, 5-dicyanopyridine-2 (1H)-thione.
Dyachenko VD and Litvinov VP.
Chemistry of Heterocyclic Compounds, 34(2), 188-194 (1998)
Dalila Rocco et al.
Molecules (Basel, Switzerland), 24(9) (2019-05-12)
The preparation of 24-functionalized 12,22:26,32-terpyridines (4'-functionalized 3,2:6',3''-terpyridines) by the reaction of three 4-alkoxybenzaldehydes with 3-acetylpyridine and ammonia was investigated; under identical reaction conditions, two (R = nC4H9, C2H5) gave the expected products whereas a third (R = nC3H7) gave only
M Jiménez et al.
Journal of agricultural and food chemistry, 49(8), 4060-4063 (2001-08-22)
A kinetic study of the inhibition of mushroom tyrosinase by 4-substituted benzaldehydes showed that these compounds behave as classical competitive inhibitors, inhibiting the oxidation of L-3,4-dihydroxyphenylalanine (L-DOPA) by mushroom tyrosinase (o-diphenolase activity). The kinetic parameter (K(I)) characterizing this inhibition was
Naoko Ueno et al.
Langmuir : the ACS journal of surfaces and colloids, 33(22), 5393-5397 (2017-05-16)
We evaluated the speed profile of self-propelled underwater oil droplets comprising a hydrophobic aldehyde derivative in terms of their diameter and the surrounding surfactant concentration using a microfluidic device. We found that the speed of the oil droplets is dependent
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.