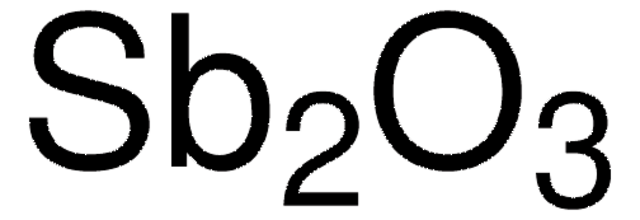230898
Antimony(III) oxide
powder, 5 μm, ReagentPlus®, 99%
Sinonimo/i:
Diantimony trioxide
Autenticatiper visualizzare i prezzi riservati alla tua organizzazione & contrattuali
About This Item
Formula condensata:
Sb2O3
Numero CAS:
Peso molecolare:
291.52
Numero CE:
Numero MDL:
Codice UNSPSC:
12352303
eCl@ss:
38080202
ID PubChem:
NACRES:
NA.23
Prodotti consigliati
Livello qualitativo
Nome Commerciale
ReagentPlus®
Saggio
99%
Forma fisica
powder
Dimensione particelle
5 μm
P. eboll.
1550 °C (lit.)
Punto di fusione
655 °C (lit.)
Stringa SMILE
O=[Sb]O[Sb]=O
InChI
1S/3O.2Sb
ADCOVFLJGNWWNZ-UHFFFAOYSA-N
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Applicazioni
- Thermal Decomposition and Carbothermal Reduction: Examines the recycling and purification processes of Antimony(III) oxide from metal oxides, valuable for those interested in sustainable metallurgy (Karlsson et al., 2018).
- Oxidation of Antimony(III) in Soil by Manganese(IV) Oxide: Studies the interaction between manganese oxide and antimony(III) oxide in soils, providing insights into environmental chemistry and soil remediation (Fu et al., 2018).
- Adsorption of Antimony(V) onto Mn(II)-Enriched Surfaces: Focuses on how manganese oxide interacts with antimony(V), relevant for those studying adsorption processes and water treatment technologies (Liu et al., 2015).
- Kinetic Modeling of Antimony(III) Oxidation and Sorption in Soils: Provides a kinetic model of antimony(III) interactions with environmental matrices, useful for environmental scientists and chemists involved in pollution control (Cai et al., 2016).
Note legali
ReagentPlus is a registered trademark of Merck KGaA, Darmstadt, Germany
Avvertenze
Warning
Indicazioni di pericolo
Consigli di prudenza
Classi di pericolo
Carc. 2
Codice della classe di stoccaggio
13 - Non Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 1
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Dispositivi di protezione individuale
Eyeshields, Gloves, type P3 (EN 143) respirator cartridges
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
P M Hext et al.
Journal of applied toxicology : JAT, 19(3), 205-209 (1999-06-11)
Diets containing antimony trioxide were fed to male and female Wistar rats of the Alpk:APSD strain over 90 days. Dose levels were 0 (control), 1000, 5000 and 20,000 ppm (equivalent to mean daily doses of 84, 421 and 1686 mg
Delia Cavallo et al.
Environmental and molecular mutagenesis, 40(3), 184-189 (2002-10-02)
The growing use of antimony (Sb) compounds in industry and the consequent increase in the number of exposed workers make it important to carry out a health risk assessment. The main goal of this study was to assess the genotoxicity
Ivo Iavicoli et al.
Journal of trace elements in medicine and biology : organ of the Society for Minerals and Trace Elements (GMS), 16(1), 33-39 (2002-03-07)
Antimony trioxide (Sb2O3) is used as a flame retardant in the textile industry. We carried out a study in a factory for the evaluation of antimony (Sb) occupational exposure and urinary levels in workers exposed to Sb2O3. Urinary levels and
R O Jenkins et al.
Human & experimental toxicology, 17(4), 231-238 (1998-06-09)
1. The aerobic filamentous fungus S. brevicaulis IMI 17297 methylated antimony from Sb2O3 substrate, with the formation of gaseous trimethylantimony (TMA). No evidence was found for the generation of other gaseous antimony compounds by this organism. 2. Biovolatilization of inorganic
Wook Jo et al.
Acta crystallographica. Section A, Foundations of crystallography, 63(Pt 3), 229-233 (2007-04-17)
The composition planes of the inversion boundary induced by the addition of Sb2O3 to ZnO ceramics were analyzed crystallographically by the application of electron back-scattered diffraction (EBSD) analysis and stereographic projection techniques. The inversion boundary was determined to consist of
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.