22110
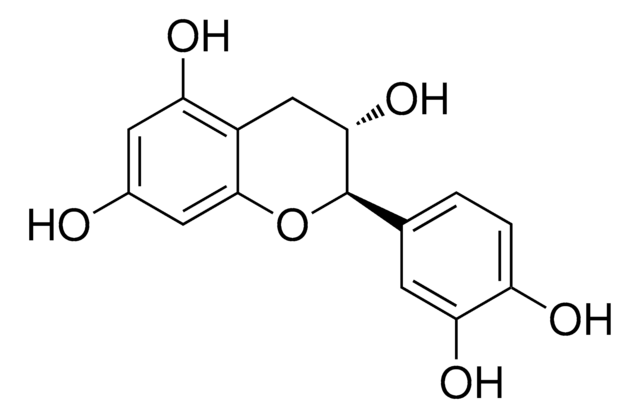
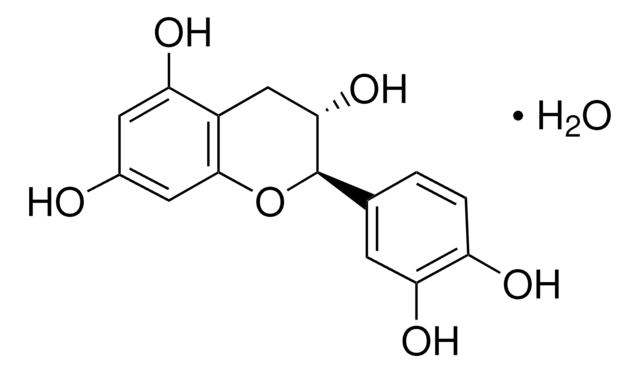
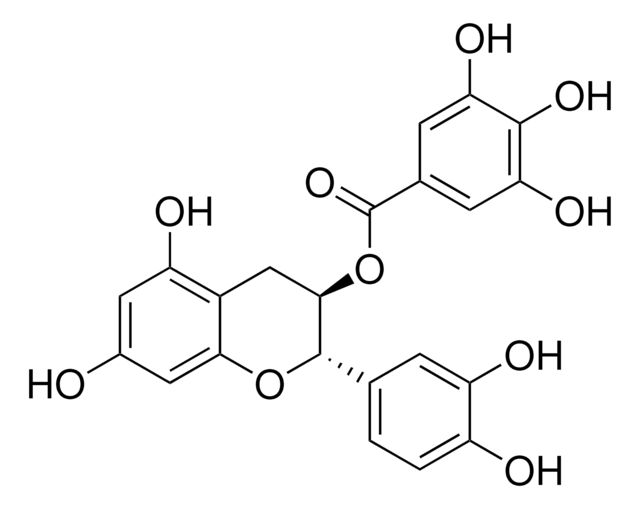
(+)-Catechin hydrate
≥96.0% (sum of enantiomers, HPLC)
Sinonimo/i:
(+)-Cyanidol-3, (2R,3S)-2-(3,4-Dihydroxyphenyl)-3,4-dihydro-1(2H)-benzopyran-3,5,7-triol
About This Item
Prodotti consigliati
Saggio
≥96.0% (sum of enantiomers, HPLC)
Forma fisica
powder
Attività ottica
[α]/D +26±2°, c = 1 in H2O
Impurezze
≤8% water
Punto di fusione
175-177 °C (anhydrous) (lit.)
Temperatura di conservazione
2-8°C
Stringa SMILE
[H]O[H].O[C@H]1Cc2c(O)cc(O)cc2O[C@@H]1c3ccc(O)c(O)c3
InChI
1S/C15H14O6.H2O/c16-8-4-11(18)9-6-13(20)15(21-14(9)5-8)7-1-2-10(17)12(19)3-7;/h1-5,13,15-20H,6H2;1H2/t13-,15+;/m0./s1
OFUMQWOJBVNKLR-NQQJLSKUSA-N
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Categorie correlate
Descrizione generale
Applicazioni
- As an inhibitor of steel corrosion in hydrochloric acid solution.
- As a model compound in the study of antimicrobial activities of flavonoids on Escherichia coli.
- As a starting material for the synthesis of catechin glucosides of biological importance.
Avvertenza
Avvertenze
Warning
Indicazioni di pericolo
Consigli di prudenza
Classi di pericolo
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Organi bersaglio
Respiratory system
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Dispositivi di protezione individuale
dust mask type N95 (US), Eyeshields, Gloves
Certificati d'analisi (COA)
Cerca il Certificati d'analisi (COA) digitando il numero di lotto/batch corrispondente. I numeri di lotto o di batch sono stampati sull'etichetta dei prodotti dopo la parola ‘Lotto’ o ‘Batch’.
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Articoli
Coumaric acid; Quercitrin; Myricetin; Quercetin
Antioxidants protect biological systems from oxidative damage produced by oxygen-containing free radicals and from redoxactive transition metal ions such as iron, copper, and cadmium.
Protocolli
Coumaric acid; Quercitrin; Myricetin; Quercetin
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.