169978
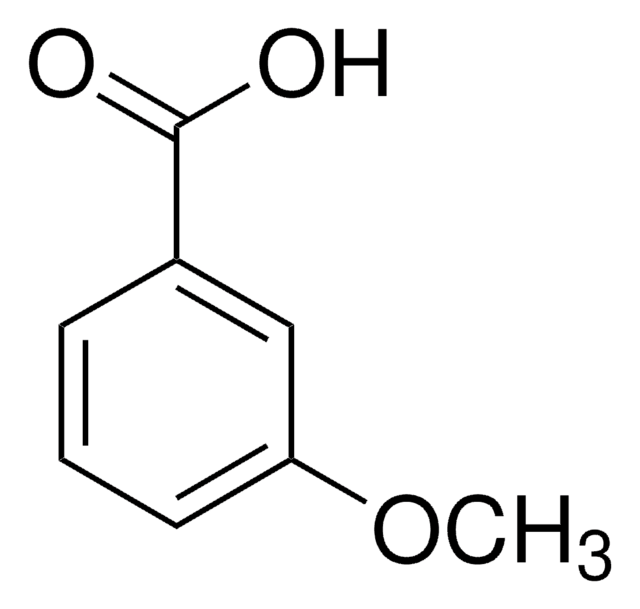
2-Methoxybenzoic acid
ReagentPlus®, 99%
Sinonimo/i:
O-Methylsalicylic acid, o-Anisic acid
Autenticatiper visualizzare i prezzi riservati alla tua organizzazione & contrattuali
About This Item
Formula condensata:
CH3OC6H4CO2H
Numero CAS:
Peso molecolare:
152.15
Beilstein:
509929
Numero CE:
Numero MDL:
Codice UNSPSC:
12352100
ID PubChem:
NACRES:
NA.22
Prodotti consigliati
Nome Commerciale
ReagentPlus®
Saggio
99%
Stato
powder
Punto di fusione
98-100 °C (lit.)
Gruppo funzionale
carboxylic acid
Stringa SMILE
COc1ccccc1C(O)=O
InChI
1S/C8H8O3/c1-11-7-5-3-2-4-6(7)8(9)10/h2-5H,1H3,(H,9,10)
ILUJQPXNXACGAN-UHFFFAOYSA-N
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Descrizione generale
2-Methoxybenzoic acid was added as carbon supplement in the culture medium of Moraxella osloensis. Photophysics of 2-methoxybenzoic acid has been investigated using both the time-correlated single photon counting and the fluorescence up-conversion techniques.
Applicazioni
2-Methoxybenzoic acid was used as internal standard during quantification of free and conjugated salicylic acid in tomato (Lycopersicon esculentum) cells by HPLC. It was also employed in the synthesis of pthalides.
Note legali
ReagentPlus is a registered trademark of Merck KGaA, Darmstadt, Germany
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
291.2 °F - closed cup
Punto d’infiammabilità (°C)
144 °C - closed cup
Dispositivi di protezione individuale
Eyeshields, Gloves, type N95 (US)
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Heterocycles, 39, 47-47 (1994)
Involvement of endogenous salicylic acid content, lipoxygenase and antioxidant enzyme activities in the response of tomato cell suspension cultures to NaCl.
Molina A, et al.
The New phytologist, 156(3), 409-415 (2002)
The photophysics of salicylic acid derivatives in aqueous solution.
Pozdnyakov IP, et al.
Journal of the Physical Society of Japan, 22(5), 449-454 (2009)
R L Crawford et al.
Journal of bacteriology, 121(3), 794-799 (1975-03-01)
Gentisate:oxygen 1,2-oxidoreductase (decyclizing) (EC 1.13.11.4; gentisate 1,2-dioxygenase) from Moraxella osloensis was purified to homogeneity as shown by polyacrylamide gel electrophoresis. The enzyme has a molecular weight of about 154,000 and gives rise to subunits of molecular weight 40,000 in the
T Sasaki et al.
Applied radiation and isotopes : including data, instrumentation and methods for use in agriculture, industry and medicine, 50(5), 905-909 (1999-04-24)
For in vivo measurement of the hydroxyl radical (.OH), we synthesized [11C]salicylic acid, [11C]O-acetylsalicylic acid and [11C]2-methoxybenzoic acid by carboxylation of 2-bromomagnesiumanisol using [11C]CO2. The radiochemical yield of [11C]salicylic acid, [11C]O-acetylsalicylic acid and [11C]2-methoxybenzoic acid calculated from trapped [11C]CO2 in
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.