13742
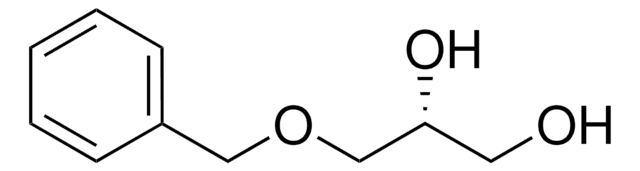
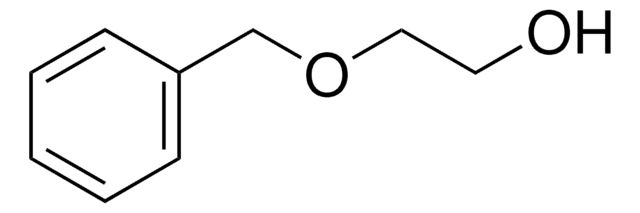
(±)-3-Benzyloxy-1,2-propanediol
≥97.0% (HPLC)
Sinonimo/i:
(±)-1-Benzylglycerol, (±)-Glycerol 1-benzyl ether
Autenticatiper visualizzare i prezzi riservati alla tua organizzazione & contrattuali
About This Item
Formula condensata:
C6H5CH2OCH2CHOHCH2OH
Numero CAS:
Peso molecolare:
182.22
Beilstein:
3199937
Numero CE:
Numero MDL:
Codice UNSPSC:
12352100
ID PubChem:
NACRES:
NA.22
Prodotti consigliati
Livello qualitativo
Saggio
≥97.0% (HPLC)
Indice di rifrazione
n20/D 1.533
Densità
1.140 g/mL at 20 °C (lit.)
Gruppo funzionale
ether
hydroxyl
phenyl
Stringa SMILE
OCC(O)COCc1ccccc1
InChI
1S/C10H14O3/c11-6-10(12)8-13-7-9-4-2-1-3-5-9/h1-5,10-12H,6-8H2
LWCIBYRXSHRIAP-UHFFFAOYSA-N
Descrizione generale
(±)-3-Benzyloxy-1,2-propanediol undergoes enantioseparation by ligand exchange micellar electrokinetic chromatography using borate anion as a central ion.
Applicazioni
(±)-3-Benzyloxy-1,2-propanediol was used in capillary electrophoretic enantioseparation of vicinol diols using different β-cyclodextrin derivatives and borate. It was used in the synthesis and immobilization of 2,3-di-O-phytanyl-sn-glycerol-1-tetraethylene glycol-(3-trichloropropyl-silane) ether lipid (DPTTC) and 2,3-di-O-phytanyl-sn-glycerol-1-tetraethylene glycol-(3-chloro-dimethylpropyl-silane) ether lipid(DPTDC).
Codice della classe di stoccaggio
10 - Combustible liquids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Dispositivi di protezione individuale
Eyeshields, Gloves
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
Vladimir Atanasov et al.
Biophysical journal, 89(3), 1780-1788 (2005-08-30)
Tethered membranes have been proven during recent years to be a powerful and flexible biomimetic platform. We reported in a previous article on the design of a new architecture based on the self-assembly of a thiolipid on ultrasmooth gold substrates
Capillary electrophoretic chiral resolution of vicinal diols by complexation with borate and cyclodextrin: Comparative studies on different cyclodextrin derivatives.
Schmid MG, et al.
Chirality, 9(2), 153-156 (1997)
Shuji Kodama et al.
Electrophoresis, 26(20), 3884-3889 (2005-09-17)
Three compounds having 1,2-diol structure (1-phenyl-1,2-ethanediol, 3-phenoxy-1,2-propanediol, and 3-benzyloxy-1,2-propanediol) were enantioseparated by ligand exchange MEKC using (5S)-pinanediol (SPD) as a chiral selector and borate anion as a central ion together with SDS. When (S)-1,2-propanediol, (S)-1,2,4-butanetriol, or (S)-3-tert-butylamino-1,2-propanediol were used as
Vincent J Huber et al.
Bioorganic & medicinal chemistry, 17(1), 411-417 (2008-01-10)
The in vitro inhibitory effects and in silico docking energies of 18 compounds with respect to aquaporin 4 (AQP4) were investigated. More than half of the compounds tested showed inhibitory activity in the in vitro functional assay and included the
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.