T4528
Thioglucosidase from Sinapis alba (white mustard) seed
≥100 units/g solid
Synonyme(s) :
Glucosinolase, Thioglucoside glucohydrolase
Se connecterpour consulter vos tarifs contractuels et ceux de votre entreprise/organisme
About This Item
Numéro CAS:
Numéro MDL:
Code UNSPSC :
12352204
Nomenclature NACRES :
NA.54
Produits recommandés
Source biologique
plant seeds (Sinapis alba)
Niveau de qualité
Forme
solid
Activité spécifique
≥100 units/g solid
Température de stockage
−20°C
Catégories apparentées
Description générale
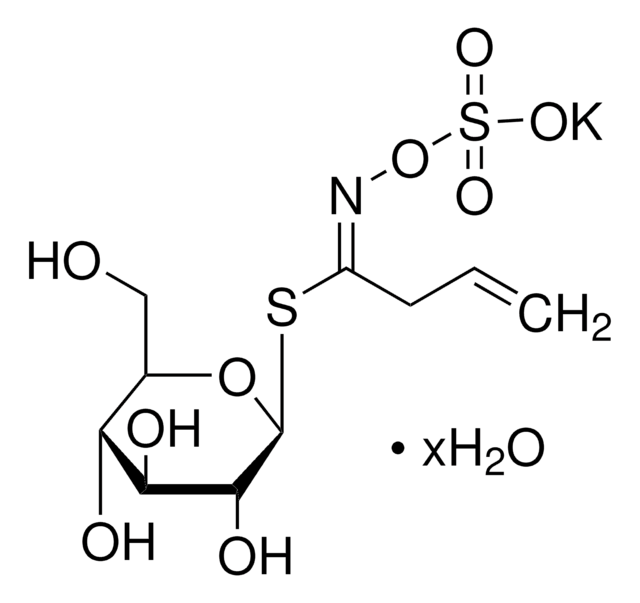
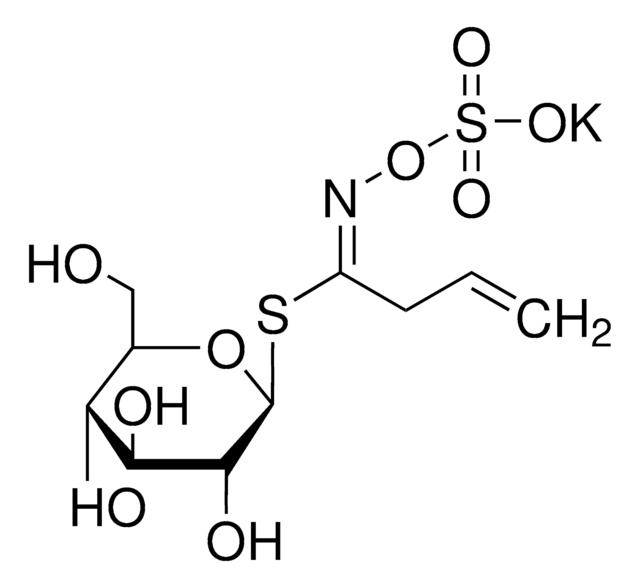
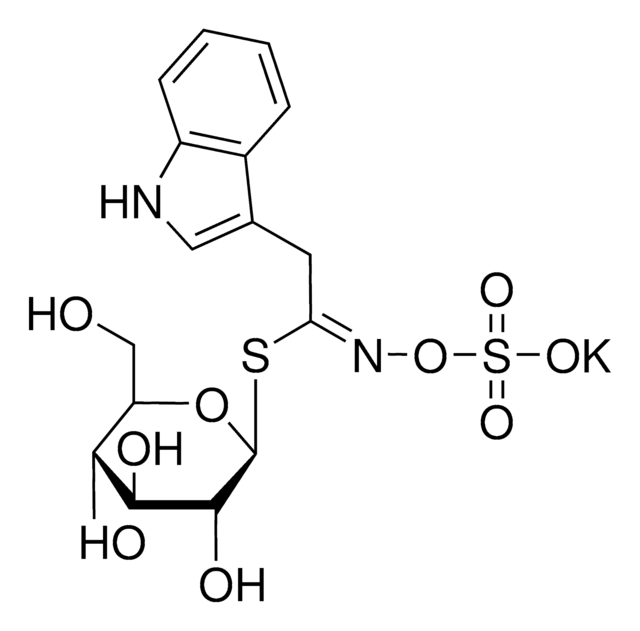
Thioglucosidase, also called as myrosinase, is present in the myrosin cells that do not contain glucosinolates. This enzyme is obtained from several plant sources, such as Lepidium sativum, L. Sinapis alba and Brassica napus.
Application
Thioglucosidase from Sinapis alba (white mustard) seeds has been used as a standard to quantify myrosinase activity and in column glucosinolate analysis of plant samples.
Thioglucosidase has been used in a study to assess Brassica species screening for glucosinolate content. Thioglucosidase has also been used in a study to investigate a negative regulatory role for auxin in sulphate deficiency response in Arabidopsis thaliana.
Actions biochimiques/physiologiques
Thioglucosidase research has focused mainly on the cruciferous crops due to their economic importance and cancer preventive benefits.
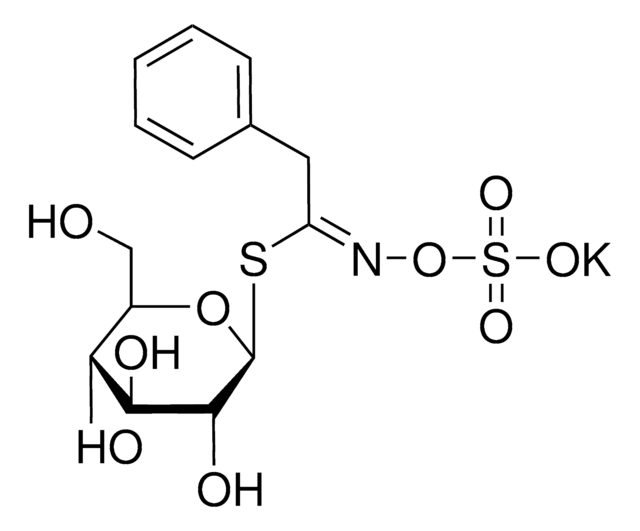
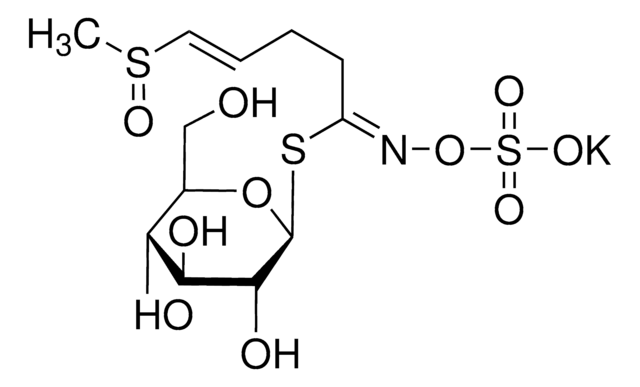
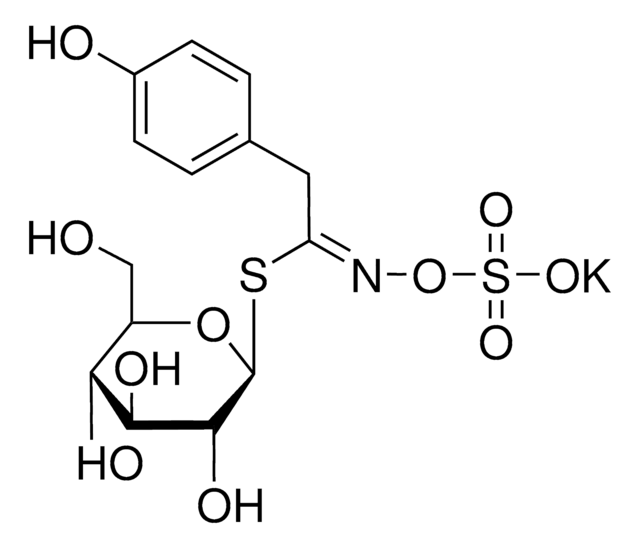
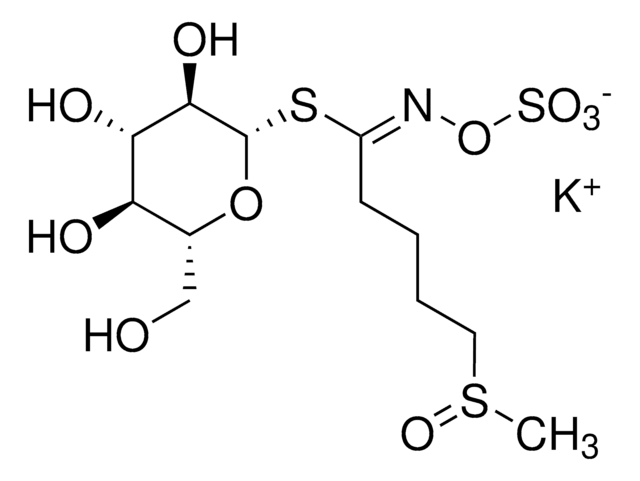
Myrosinases are present in many bacteria, fungi, and edible plants, including those of the Brassicaceae (Cruciferae) family. The enzyme hydrolyzes the S-glucosidic bond of a glucosinolate substrate to form an unstable aglycone that rearranges with the loss of sulfate primarily to the isothiocyanate, though thiocyanates and nitriles are also formed. Many of the isothiocyanate products of aliphatic and aromatic glucosinolates have cancer chemopreventive properties.
Définition de l'unité
One unit will produce 1.0 μmole glucose per min from sinigrin at pH 6.0 at 25 °C.
Mention d'avertissement
Danger
Mentions de danger
Conseils de prudence
Classification des risques
Resp. Sens. 1
Code de la classe de stockage
11 - Combustible Solids
Classe de danger pour l'eau (WGK)
WGK 3
Point d'éclair (°F)
Not applicable
Point d'éclair (°C)
Not applicable
Équipement de protection individuelle
Eyeshields, Gloves, type N95 (US)
Certificats d'analyse (COA)
Recherchez un Certificats d'analyse (COA) en saisissant le numéro de lot du produit. Les numéros de lot figurent sur l'étiquette du produit après les mots "Lot" ou "Batch".
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Les clients ont également consulté
Palatability of Thlaspi caerulescens for snails: influence of zinc and glucosinolates
Noret N, et al.
The New phytologist, 165(3), 763-772 (2005)
Bioavailability of Isothiocyanates From Broccoli Sprouts in Protein, Lipid, and Fiber Gels
Oliviero T, et al.
Molecular Nutrition And Food Research, 62(18), 1700837-1700837 (2018)
Si-Jun Zheng et al.
Journal of chemical ecology, 37(8), 818-829 (2011-06-22)
The jasmonic acid (JA) signaling pathway and defensive secondary metabolites such as glucosinolates are generally considered to play central roles in the defense of brassicaceous plants against herbivorous insects. To determine the function of specific plant genes in plant-insect interactions
Elena Peñas et al.
Journal of agricultural and food chemistry, 59(8), 3772-3779 (2011-03-19)
The influence of two Spanish growing locations with well-differentiated climatic conditions (northern and eastern areas) on the main bioactive compounds, glucosinolates (GLS), total phenolic compounds (TPC), and vitamin C, as well as myrosinase activity and antioxidant capacity in five white
Chemical defence and toxins of plants
Yamane H, et al.
Journal of Hospital Infection (2010)
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique