SML2200
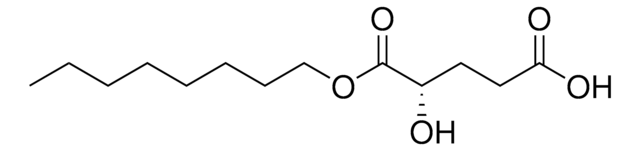
Octyl-(R)-2HG
≥98% (HPLC), film, α-KG-dependent dioxygenases prolyl hydroxylases inhibitor
Synonyme(s) :
(2R)-2-Hydroxyglutarate octyl ester, (2R)-Octyl-α-hydroxyglutarate, 1-Octyl-D-2-hydroxyglutarate, 1-octyl ester, 2R-Hydroxy-pentanedioic acid, Octyl-2HG, Octyl-D-2HG, R-2HG octyl ester
About This Item
Produits recommandés
Nom du produit
Octyl-(R)-2HG, ≥98% (HPLC)
Essai
≥98% (HPLC)
Forme
film
Conditions de stockage
desiccated
Couleur
colorless
Solubilité
DMSO: 2 mg/mL, clear
Température de stockage
−20°C
Chaîne SMILES
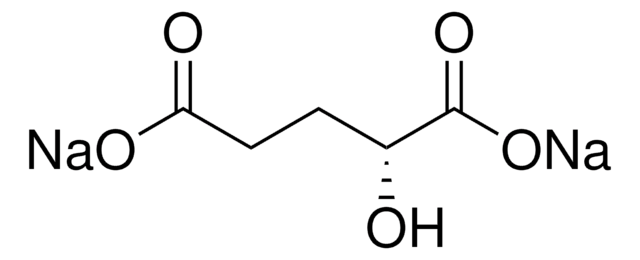
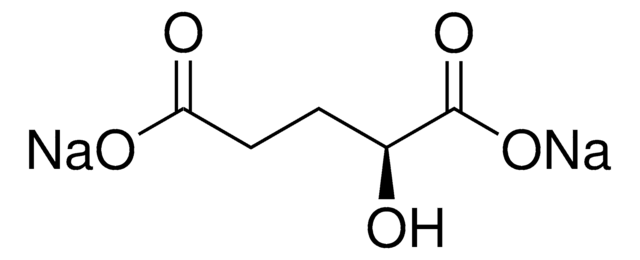
O[C@H](CCC(=O)O)C(=O)OCCCCCCCC
Clé InChI
UJZOKTKSGUOCCM-LLVKDONJSA-N
Application
Actions biochimiques/physiologiques
Notes préparatoires
Code de la classe de stockage
11 - Combustible Solids
Classe de danger pour l'eau (WGK)
WGK 3
Point d'éclair (°F)
Not applicable
Point d'éclair (°C)
Not applicable
Faites votre choix parmi les versions les plus récentes :
Certificats d'analyse (COA)
It looks like we've run into a problem, but you can still download Certificates of Analysis from our Documents section.
Si vous avez besoin d'assistance, veuillez contacter Service Clients
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Les clients ont également consulté
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique