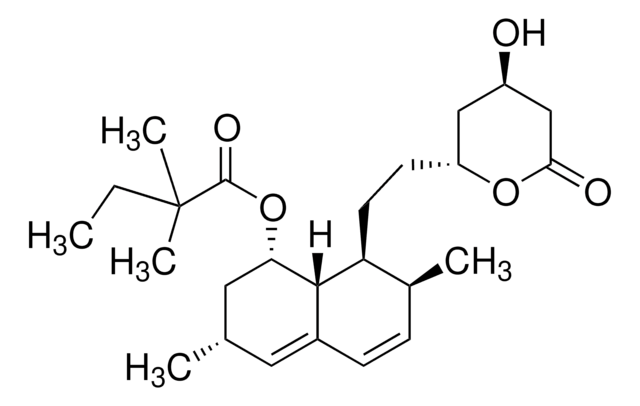
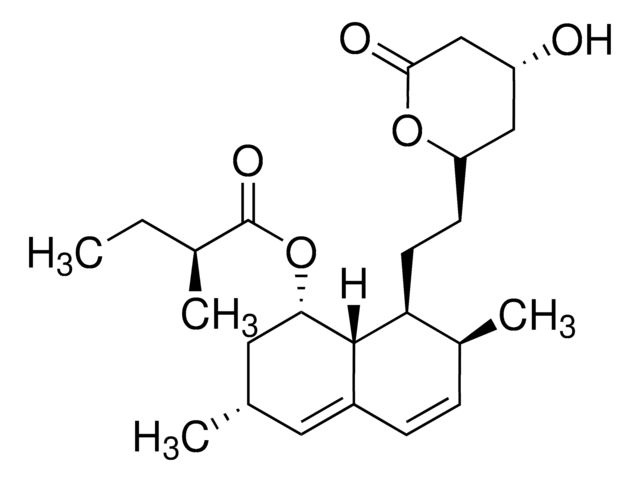
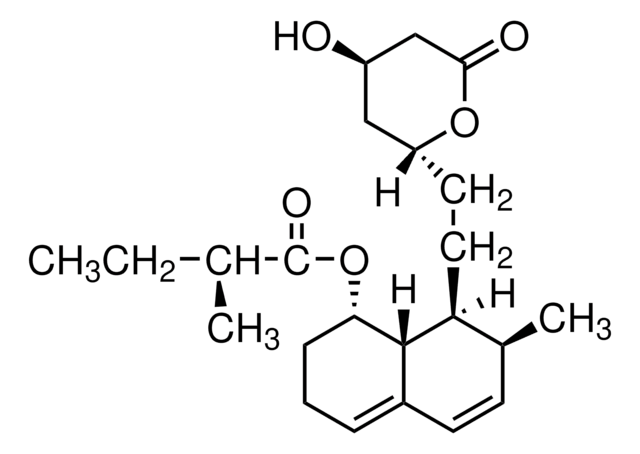
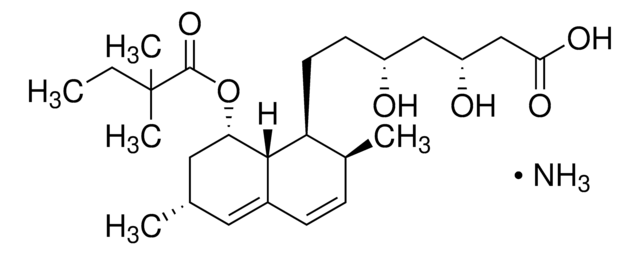
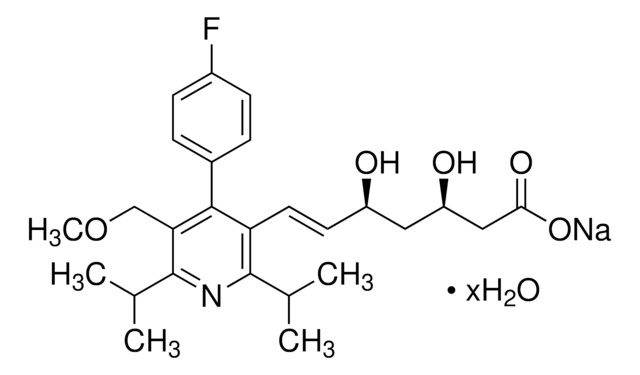
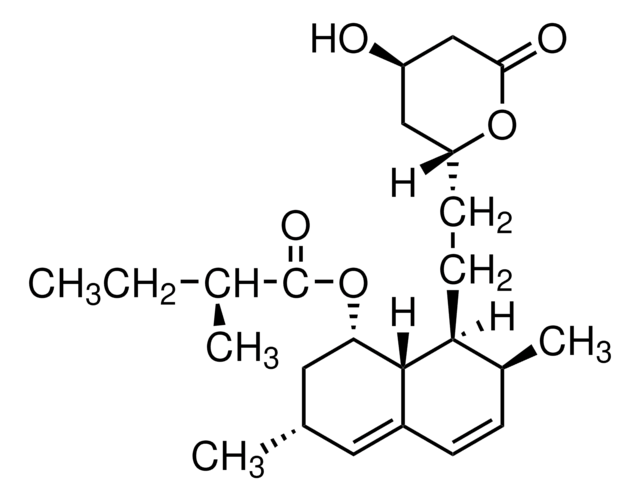
S6196
Simvastatin
≥97% (HPLC), solid
Synonyme(s) :
MK-733, SVA
About This Item
Produits recommandés
Pureté
≥97% (HPLC)
Forme
solid
Couleur
white
Pf
127-132 °C (lit.)
Solubilité
DMSO: ≥20 mg/mL
Auteur
Merck & Co., Inc., Kenilworth, NJ, U.S.
Température de stockage
2-8°C
Chaîne SMILES
[H][C@]12[C@H](C[C@@H](C)C=C1C=C[C@H](C)[C@@H]2CC[C@@H]3C[C@@H](O)CC(=O)O3)OC(=O)C(C)(C)CC
InChI
1S/C25H38O5/c1-6-25(4,5)24(28)30-21-12-15(2)11-17-8-7-16(3)20(23(17)21)10-9-19-13-18(26)14-22(27)29-19/h7-8,11,15-16,18-21,23,26H,6,9-10,12-14H2,1-5H3/t15-,16-,18+,19+,20-,21-,23-/m0/s1
Clé InChI
RYMZZMVNJRMUDD-HGQWONQESA-N
Informations sur le gène
human ... HMGCR(3156)
rat ... Hmgcr(25675)
Vous recherchez des produits similaires ? Visite Guide de comparaison des produits
Description générale
Application
- as an inhibitor of HMG CoA reductase (HMGCR)
- to study its effects on epithelial to mesenchymal transition (EMT) and the prognosis of patients with lung adenocarcinoma
- in in vivo studies to test its effect on brain tumor−initiating cells (BTIC) viability and cell proliferation
- to study the role of adenosine triphosphate (ATP)-binding cassette transporter A7 in phagocytosis of Jurkat cells
- to study the effect on endothelial dysfunction and inflammation in mice
Actions biochimiques/physiologiques
Caractéristiques et avantages
Mention d'avertissement
Warning
Mentions de danger
Conseils de prudence
Classification des risques
Repr. 2
Code de la classe de stockage
11 - Combustible Solids
Classe de danger pour l'eau (WGK)
WGK 3
Point d'éclair (°F)
Not applicable
Point d'éclair (°C)
Not applicable
Équipement de protection individuelle
Eyeshields, Gloves, type N95 (US)
Certificats d'analyse (COA)
Recherchez un Certificats d'analyse (COA) en saisissant le numéro de lot du produit. Les numéros de lot figurent sur l'étiquette du produit après les mots "Lot" ou "Batch".
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Les clients ont également consulté
Articles
Dietary Terpenes
The amount of cholesterol that is synthesized in the liver is tightly regulated by dietary cholesterol levels. LDL receptors regulate the cellular transport of lipid rich low density lipoprotein (LDL) particles.
Randomized controlled clinical studies have suggested 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase inhibitors (statins) are effective in both primary and secondary prevention of cardiovascular disease (CVD) events.
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique