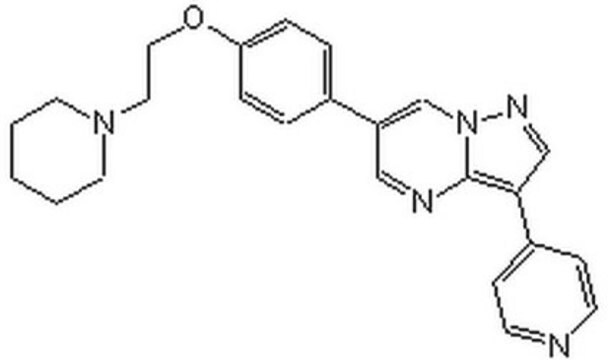
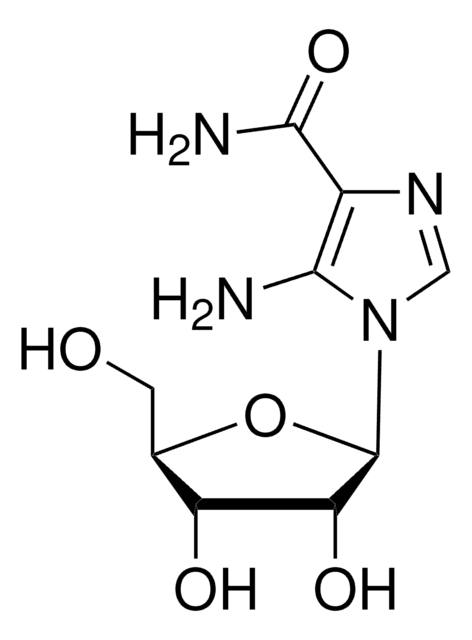
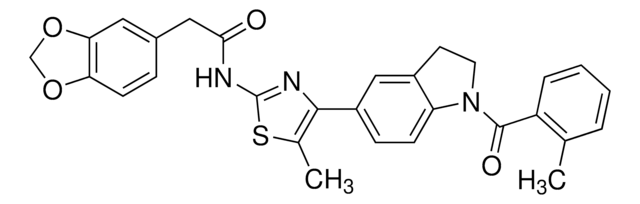
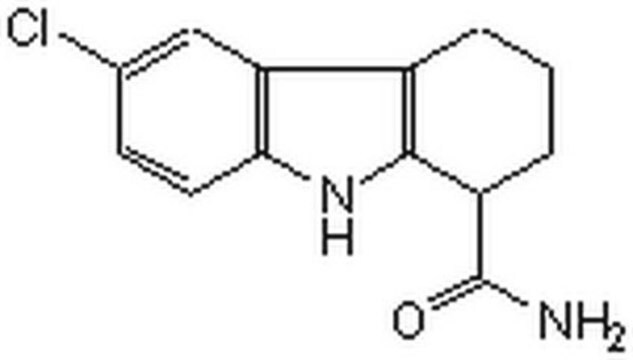
E7034
EX-527
≥98% (HPLC)
Synonyme(s) :
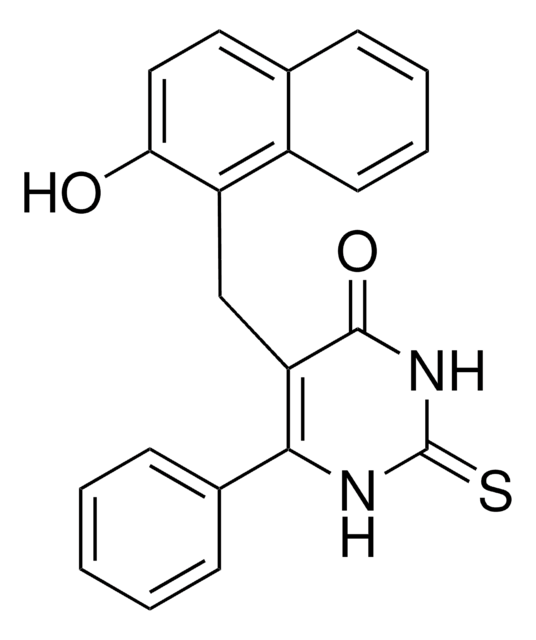
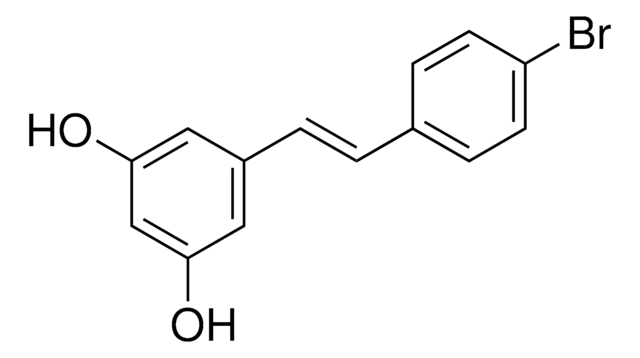
6-Chloro-2,3,4,9-tetrahydro-1H-Carbazole-1-carboxamide
About This Item
Produits recommandés
Essai
≥98% (HPLC)
Forme
powder
Couleur
white to beige
Solubilité
DMSO: >20 mg/mL
Température de stockage
2-8°C
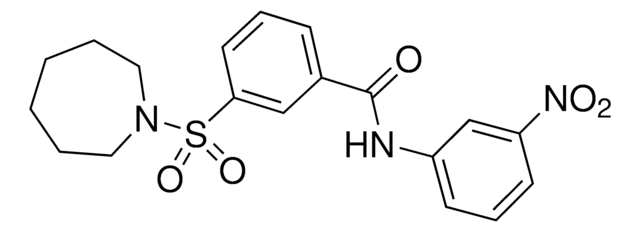
Chaîne SMILES
NC(=O)C1CCCc2c1[nH]c3ccc(Cl)cc23
InChI
1S/C13H13ClN2O/c14-7-4-5-11-10(6-7)8-2-1-3-9(13(15)17)12(8)16-11/h4-6,9,16H,1-3H2,(H2,15,17)
Clé InChI
FUZYTVDVLBBXDL-UHFFFAOYSA-N
Application
- in 1% dimethyl sulfoxide, 30%, polyethylene glycol-400 and 1% Tween 80 for treating C57BL/6 N mice to study its effect on intestinal morphological changes and crypt cell apoptosis
- as a an inhibitor of sirtuin 1, in treating human cancer lines MCF-7 (Michigan cancer foundation-7) and HCT116 (colon cancer cell line) incubated in Dulbecco′s modified Eagle′s medium, to study its effect on mitochondrial ATP (adenosine triphosphate) production
- Intracerebroventricularly infused in rat model of epileptogenesis, to access kainic acid–induced status epilepticus stimulated sirtuin 1 activity
Actions biochimiques/physiologiques
Caractéristiques et avantages
Produit(s) apparenté(s)
Code de la classe de stockage
11 - Combustible Solids
Classe de danger pour l'eau (WGK)
WGK 3
Faites votre choix parmi les versions les plus récentes :
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Les clients ont également consulté
Articles
Epigenetic modifications are thought to occur through two key interconnected processes—DNA methylation and the covalent modification of histones.
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique