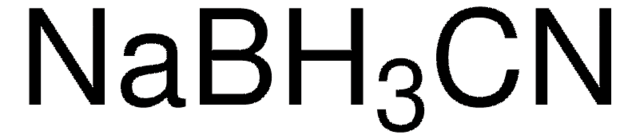C4187
Cyanoborohydride Coupling Buffer
Synonyme(s) :
Coupling buffer
Se connecterpour consulter vos tarifs contractuels et ceux de votre entreprise/organisme
About This Item
Code UNSPSC :
12161700
Nomenclature NACRES :
NA.56
Produits recommandés
Forme
liquid
Niveau de qualité
Pertinence de la réaction
reagent type: reductant
Technique(s)
affinity chromatography: suitable
Application(s)
life science and biopharma
Température de stockage
2-8°C
Application
A ready-to-use reagent used to couple amine ligands to aldehyde functional groups. The coupling buffer reaction is a reductive amination of the intermediate Schiff′s base to a stable C−N bond.
Cyanoborohydride Coupling Buffer has been used:
- in coupling reactions between amines and glutaraldehyde
- to reduce hydrazone bond to a stable hydrazide bond
- as a component in oligonucleotide reaction mixture for coverslips functionalization
Cyanoborohydride Coupling Buffer is used in affinity chromatography, protein chromatography, activated/functionalized matrices and synthetic reagents. Cyanoborohydride has been used to inform a safe and effective gene-transfer system targeting hepatocytes as well as to develop a method for targeted delivery of anticancer therapeutics to cancer cells in hypoxic areas.
Actions biochimiques/physiologiques
Cyanoborohydride Coupling Buffer is a reagent suitable for reductive amination processes, that contributes to transformation of simple alcohols into more complex amines. It is used in the conversion of Schiff base, by reducing it, to form a secondary amine without affecting aldehyde groups on the support.
Composants
0.02 M sodium phosphate, pH 7.5, containing 0.2 M sodium chloride and 3.0 g/L sodium cyanoborohydride
Mention d'avertissement
Warning
Mentions de danger
Conseils de prudence
Classification des risques
Acute Tox. 4 Oral - Aquatic Chronic 2
Code de la classe de stockage
12 - Non Combustible Liquids
Classe de danger pour l'eau (WGK)
WGK 3
Point d'éclair (°F)
Not applicable
Point d'éclair (°C)
Not applicable
Faites votre choix parmi les versions les plus récentes :
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Use of polymer supported reagents for clean multi-step organic synthesis: preparation of amines and amine derivatives from alcohols for use in compound library generation
Ley S, et al.
Journal of the Chemical Society. Perkin Transactions 1, 15(27), 2239-2242 (1998)
Christopher A Holden et al.
International journal of nanomedicine, 5, 25-36 (2010-02-18)
Tumors frequently contain hypoxic regions that result from a shortage of oxygen due to poorly organized tumor vasculature. Cancer cells in these areas are resistant to radiation- and chemotherapy, limiting the treatment efficacy. Macrophages have inherent hypoxia-targeting ability and hold
Direct electrical detection of antigen?antibody binding on diamond and silicon substrates using electrical impedance spectroscopy.
Yang W, et al.
Analyst, 132(4), 296-306 (2007)
Binding between the integrin ?X?2 (CD11c/CD18) and heparin.
Vorup-Jensen T, et al.
Test, 282(42), 30869-30877 (2007)
Polymer-supported triacetoxyborohydride: a novel reagent of choice for reductive amination
Bhattacharyya S, et al.
Tetrahedron Letters, 44(27), 4957-4960 (2003)
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique