PHR1928
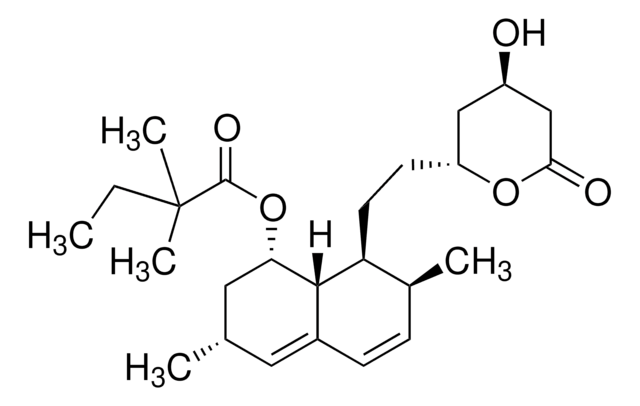
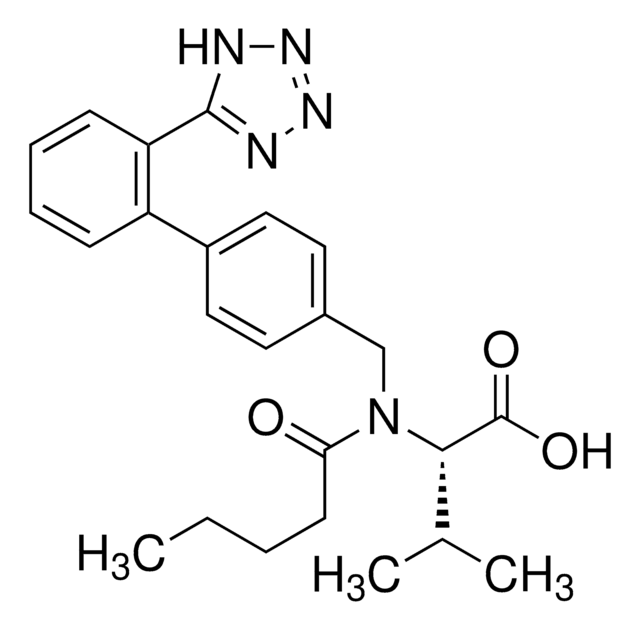
Rosuvastatin Calcium
Pharmaceutical Secondary Standard; Certified Reference Material
Synonyme(s) :
Rosuvastatin calcium salt
About This Item
Produits recommandés
Qualité
certified reference material
pharmaceutical secondary standard
Niveau de qualité
Agence
traceable to Ph. Eur. Y0001719
traceable to USP 1606015
Famille d'API
rosuvastatin
Forme
powder
CofA (certificat d'analyse)
current certificate can be downloaded
Conditionnement
pkg of 1 g
Application(s)
pharmaceutical
Température de stockage
-10 to -25°C
InChI
1S/2C22H28FN3O6S.Ca/c2*1-13(2)20-18(10-9-16(27)11-17(28)12-19(29)30)21(14-5-7-15(23)8-6-14)25-22(24-20)26(3)33(4,31)32;/h2*5-10,13,16-17,27-28H,11-12H2,1-4H3,(H,29,30);/q;;+2/p-2/b2*10-9+;/t2*16-,17-;/m11./s1
Clé InChI
LALFOYNTGMUKGG-BGRFNVSISA-L
Vous recherchez des produits similaires ? Visite Guide de comparaison des produits
Description générale
Pharmaceutical secondary standards for application in quality control provide pharma laboratories and manufacturers with a convenient and cost-effective alternative to the preparation of in-house working standards.
Rosuvastatin Calcium, an HMG-CoA reductase inhibitor, belongs to the statins class of pharmaceuticals and is used for the treatment of hypercholesterolemia.
Application
- Spectrophotometric estimation of rosuvastatin calcium in its pure form and tablet formulations
- Determination of rosuvastatin calcium by square-wave voltammetry using a boron-doped diamond electrode in two tablet samples and biological fluid samples of human urine and serum
- Simultaneous quantification of rosuvastatin and amlodipine by high-performance liquid chromatography (HPLC) in combined pharmaceutical formulations
- Determination of rosuvastatin calcium and related substances in tablets of rosuvastatin using a reversed-phase high-performance liquid chromatographic (RP-HPLC) method
Remarque sur l'analyse
Note de bas de page
Produits recommandés
Produit(s) apparenté(s)
Code de la classe de stockage
11 - Combustible Solids
Classe de danger pour l'eau (WGK)
WGK 3
Point d'éclair (°F)
Not applicable
Point d'éclair (°C)
Not applicable
Faites votre choix parmi les versions les plus récentes :
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Les clients ont également consulté
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique