424633
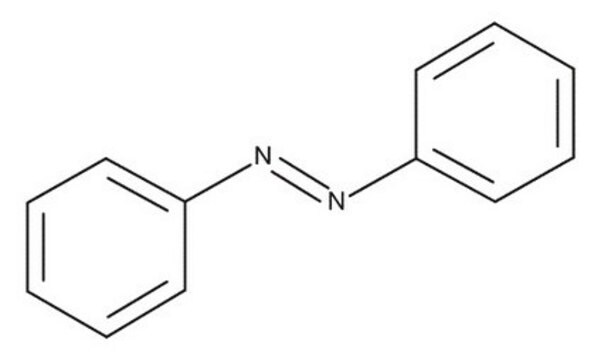
Azobenzene
98%
Synonyme(s) :
1,2-Diphenyldiazene; Diphenyldiazene
About This Item
Produits recommandés
Pression de vapeur
1 mmHg ( 104 °C)
Niveau de qualité
Pureté
98%
Forme
powder or crystals
Température d'inflammation spontanée
890 °F
Point d'ébullition
293 °C (lit.)
Pf
65-68 °C (lit.)
Densité
1.09 g/mL at 25 °C (lit.)
Application(s)
diagnostic assay manufacturing
hematology
histology
Température de stockage
room temp
Chaîne SMILES
c1ccc(cc1)\N=N\c2ccccc2
InChI
1S/C12H10N2/c1-3-7-11(8-4-1)13-14-12-9-5-2-6-10-12/h1-10H/b14-13+
Clé InChI
DMLAVOWQYNRWNQ-BUHFOSPRSA-N
Vous recherchez des produits similaires ? Visite Guide de comparaison des produits
Catégories apparentées
Description générale
Application
As human tissue is translucent to red and near-infrared light but opaque to blue and UV light, Azobenzene is important in medicine and photopharmacology for applications that involve shifting the absorptions of both trans and cis isomers of azobenzene to longer wavelengths.
Mention d'avertissement
Danger
Mentions de danger
Conseils de prudence
Classification des risques
Acute Tox. 4 Inhalation - Acute Tox. 4 Oral - Aquatic Acute 1 - Aquatic Chronic 1 - Carc. 1B - Muta. 2 - STOT RE 2
Code de la classe de stockage
6.1C - Combustible, acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
Classe de danger pour l'eau (WGK)
WGK 3
Point d'éclair (°F)
212.0 °F - closed cup
Point d'éclair (°C)
100.0 °C - closed cup
Équipement de protection individuelle
Eyeshields, Gloves, type P3 (EN 143) respirator cartridges
Choose from one of the most recent versions:
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Les clients ont également consulté
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique