39303
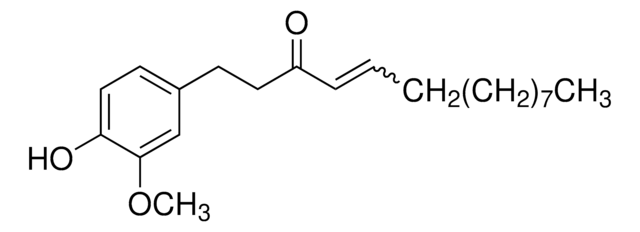
[6]-Shogaol
analytical standard
Synonyme(s) :
1-(4-Hydroxy-3-methoxyphenyl)-4-decen-3-one
About This Item
Produits recommandés
Qualité
analytical standard
Niveau de qualité
Essai
≥90.0% (HPLC)
Durée de conservation
limited shelf life, expiry date on the label
Technique(s)
HPLC: suitable
gas chromatography (GC): suitable
Application(s)
food and beverages
Format
neat
Température de stockage
2-8°C
Chaîne SMILES
CCCCC\C=C\C(=O)CCc1ccc(O)c(OC)c1
InChI
1S/C17H24O3/c1-3-4-5-6-7-8-15(18)11-9-14-10-12-16(19)17(13-14)20-2/h7-8,10,12-13,19H,3-6,9,11H2,1-2H3/b8-7+
Clé InChI
OQWKEEOHDMUXEO-BQYQJAHWSA-N
Vous recherchez des produits similaires ? Visite Guide de comparaison des produits
Description générale
Application
Conditionnement
Autres remarques
Produits recommandés
Faites votre choix parmi les versions les plus récentes :
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Les clients ont également consulté
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
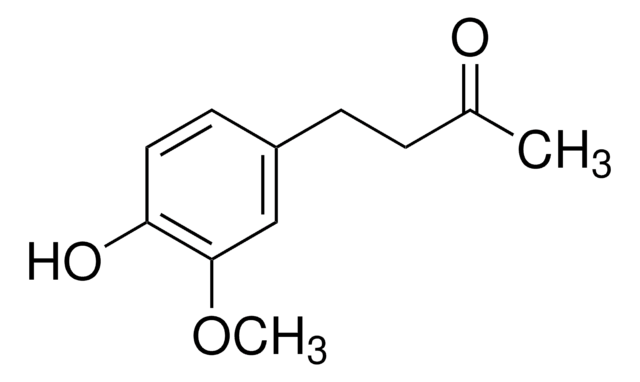
Contacter notre Service technique![[6]-Gingerol analytical standard](/deepweb/assets/sigmaaldrich/product/structures/259/444/6877889c-1cc0-47f5-b807-f847deadec1d/640/6877889c-1cc0-47f5-b807-f847deadec1d.png)
![[6]-Gingerol, Zingiber officinale An antitumor, apoptosis-inducing compound of the ginger family that blocks EGF-induced cell transformation by inhibiting the activation of Activator Protein-1 (AP-1).](/deepweb/assets/sigmaaldrich/product/images/140/919/0846df46-0b67-4c28-b99d-87e177be65b2/640/0846df46-0b67-4c28-b99d-87e177be65b2.jpg)
![[10]-Gingerol ≥98% (HPLC)](/deepweb/assets/sigmaaldrich/product/structures/224/210/b4f3e699-03b9-4112-89c1-a63f196344d0/640/b4f3e699-03b9-4112-89c1-a63f196344d0.png)
![[8]-Gingerol analytical standard](/deepweb/assets/sigmaaldrich/product/structures/408/530/af2f2837-3419-4e07-a72b-24e95af0d7ce/640/af2f2837-3419-4e07-a72b-24e95af0d7ce.png)