16101
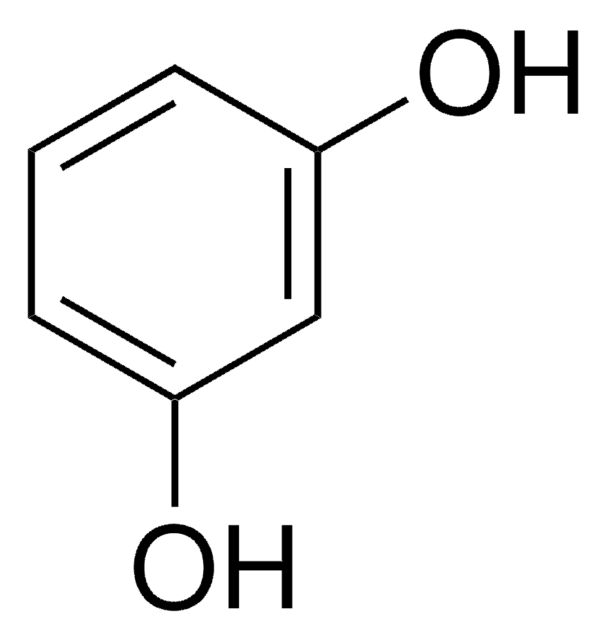
Resorcinol
meets analytical specification of Ph. Eur., BP, 98.5-100.5% (calc. to the dried substance)
Synonyme(s) :
1,3-Benzenediol
About This Item
Densité de vapeur
3.8 (vs air)
Niveau de qualité
Pression de vapeur
1 mmHg ( 21.1 °C)
Pureté
98.5-100.5% (calc. to the dried substance)
Forme
solid
Température d'inflammation spontanée
1126 °F
Qualité
meets analytical specification of Ph. Eur., BP
Impuretés
acidity or alkalinity, complies
related subst., complies
residual solvents, complies
≤0.001% heavy metals (as Pb)
≤0.01% free acid (as H2SO4)
≤0.01% free alkali (as NH3)
≤0.01% pyrocatechol
Résidus de calcination
≤0.05% (as SO4)
Perte
≤1.0% loss on drying (on silica gel)
Point d'ébullition
178 °C/16 mmHg (lit.)
Pf
109-112 °C (lit.)
Solubilité
water: soluble
Traces d'anions
chloride (Cl-): ≤100 mg/kg
sulfate (SO42-): ≤500 mg/kg
Adéquation
complies for appearance of solution
complies for identity
Application(s)
pharmaceutical (small molecule)
Chaîne SMILES
Oc1cccc(O)c1
InChI
1S/C6H6O2/c7-5-2-1-3-6(8)4-5/h1-4,7-8H
Clé InChI
GHMLBKRAJCXXBS-UHFFFAOYSA-N
Vous recherchez des produits similaires ? Visite Guide de comparaison des produits
Application
Actions biochimiques/physiologiques
Mention d'avertissement
Danger
Mentions de danger
Conseils de prudence
Classification des risques
Acute Tox. 4 Oral - Aquatic Acute 1 - Aquatic Chronic 3 - Eye Dam. 1 - Skin Irrit. 2 - Skin Sens. 1B - STOT SE 1 Oral - STOT SE 2 Oral
Organes cibles
Central nervous system,Blood, Respiratory system
Code de la classe de stockage
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
Classe de danger pour l'eau (WGK)
WGK 2
Point d'éclair (°F)
260.6 °F - closed cup
Point d'éclair (°C)
127 °C - closed cup
Équipement de protection individuelle
dust mask type N95 (US), Eyeshields, Faceshields, Gloves
Choose from one of the most recent versions:
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Les clients ont également consulté
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique