08902
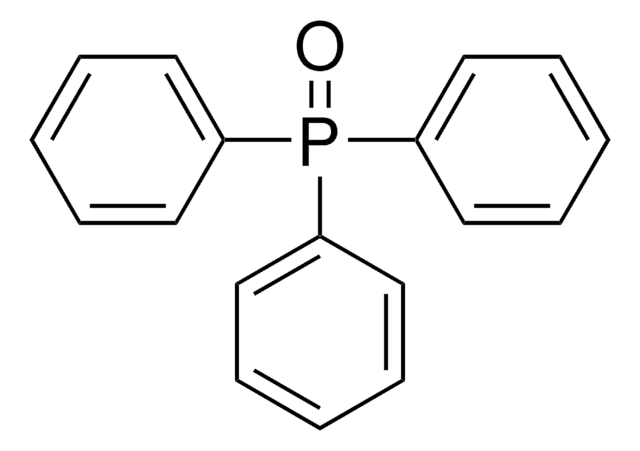
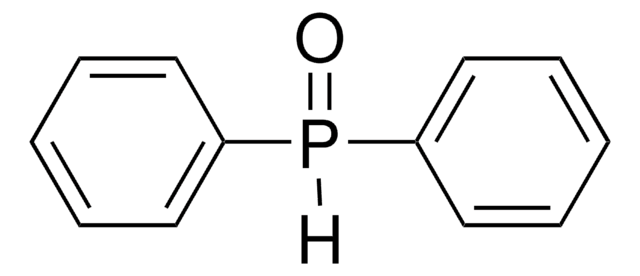
Triphenylphosphine oxide
analytical standard
Synonyme(s) :
Ph3PO, TPPO, Triphenyl phosphorus oxide, Triphenylphosphine monoxide
About This Item
Produits recommandés
Qualité
analytical standard
Niveau de qualité
Pureté
≥98.5% (HPLC)
Durée de conservation
limited shelf life, expiry date on the label
Technique(s)
HPLC: suitable
gas chromatography (GC): suitable
Pf
150-157 °C (lit.)
Application(s)
pharmaceutical
Format
neat
Chaîne SMILES
O=P(c1ccccc1)(c2ccccc2)c3ccccc3
InChI
1S/C18H15OP/c19-20(16-10-4-1-5-11-16,17-12-6-2-7-13-17)18-14-8-3-9-15-18/h1-15H
Clé InChI
FIQMHBFVRAXMOP-UHFFFAOYSA-N
Vous recherchez des produits similaires ? Visite Guide de comparaison des produits
Application
- Fluoride sensing in coordination polymers: Triphenylphosphine oxide is utilized in the development of lanthanide-based coordination polymers for selective fluoride sensing, demonstrating its utility in intramolecular proton transfer mechanisms for enhanced detection capabilities (Singh et al., 2024).
- NMR shielding calculations: The compound′s influence on solvent effects and convergence of phosphorus-31 NMR shielding calculations is explored, highlighting its role in computational chemistry for better understanding molecular interactions (Calcagno et al., 2024).
- Hydrogen bond characterization: Triphenylphosphine oxide is investigated for its interactions with substituted phenols, where shifts in phosphorus-oxygen stretching bands are analyzed to characterize hydrogen bonds, enhancing our understanding of molecular dynamics in phosphine oxides (Kostin et al., 2024).
- Advances in utilization: A review discusses the broad applications of triphenylphosphine oxide beyond its traditional uses, focusing on its emerging roles in various chemical transformations and its potential in catalytic processes (Zhang and Han, 2024).
- Covalent bonding studies with lanthanides: The role of 4f-orbitals in forming covalent bonds between lanthanides and triphenylphosphine oxide is examined using X-ray absorption spectroscopy and density functional theory, providing insights into the electronic structure and bonding behavior in these complexes (Zhang et al., 2024).
Remarque sur l'analyse
Autres remarques
The collision cross section (CCS) measurement was provided by Waters Corporation, using the SYNAPT XS mass spectrometer.
For a description and overview of how ion mobility enables the measurement of the CCS of an ion visit ims.waters.com.
Further information on the SYNAPT XS mass spectrometer can be found on the IMS microsite and product webpage.
TWCCS measurements are expected to be within 2% of this reference value.
P/N 08902 is part of the Waters Extractables & Leachables UNIFI scientific library which can be downloaded from Waters Marketplace.
Produits recommandés
Mention d'avertissement
Warning
Mentions de danger
Conseils de prudence
Classification des risques
Acute Tox. 4 Oral - Aquatic Chronic 3
Code de la classe de stockage
11 - Combustible Solids
Classe de danger pour l'eau (WGK)
WGK 2
Point d'éclair (°F)
356.0 °F
Point d'éclair (°C)
180 °C
Faites votre choix parmi les versions les plus récentes :
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique