T-058
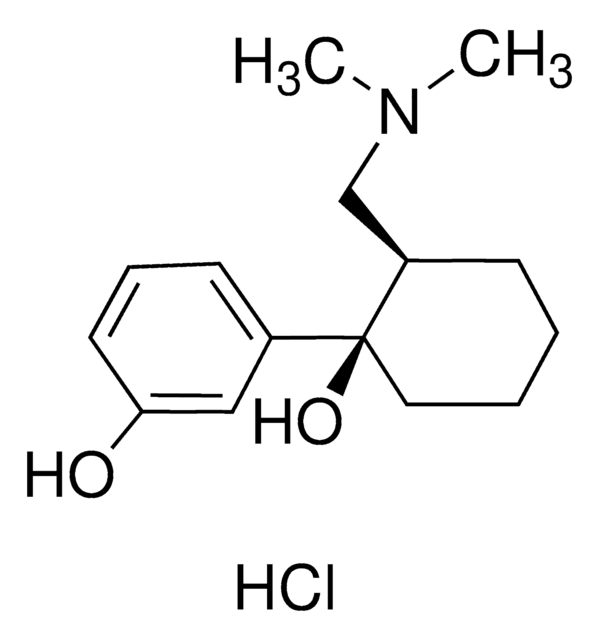
Tapentadol hydrochloride solution
1.0 mg/mL in methanol (as free base), ampule of 1 mL, certified reference material, Cerilliant®
Synonyme(s) :
Tapentadol hydrochloride solution
About This Item
Produits recommandés
Qualité
certified reference material
Forme
liquid
Caractéristiques
Snap-N-Spike®/Snap-N-Shoot®
Conditionnement
ampule of 1 mL
Fabricant/nom de marque
Cerilliant®
drug control
Narcotic Licence Schedule A (Switzerland); estupefaciente (Spain); Decreto Lei 15/93: Tabela IA (Portugal)
Concentration
1.0 mg/mL in methanol (as free base)
Technique(s)
gas chromatography (GC): suitable
liquid chromatography (LC): suitable
Application(s)
forensics and toxicology
Format
single component solution
Température de stockage
−20°C
Chaîne SMILES
Cl.CC[C@H]([C@@H](C)CN(C)C)c1cccc(O)c1
InChI
1S/C14H23NO.ClH/c1-5-14(11(2)10-15(3)4)12-7-6-8-13(16)9-12;/h6-9,11,14,16H,5,10H2,1-4H3;1H/t11-,14+;/m0./s1
Clé InChI
ZELFLGGRLLOERW-YECZQDJWSA-N
Informations sur le gène
human ... OPRM1(4988) , SLC6A2(6530)
Description générale
Application
- Extended-Release Formulation for Chronic Pain: Extended-release formulations of Tapentadol hydrochloride are developed to provide sustained pain relief for chronic conditions. This formulation reduces the frequency of dosing and enhances patient compliance, offering consistent pain management (Faria et al., 2017).
- Pharmaceutical Quality Control and Research: High-purity Tapentadol hydrochloride solutions are essential in pharmaceutical research and quality control. These solutions are used for the calibration of analytical instruments and ensuring the consistency and safety of pharmaceutical products (Fejos et al., 2014).
- Transdermal Delivery Systems: Innovative research has developed PEGylated ultra-deformable transferosomes for the transdermal delivery of Tapentadol, improving its bioavailability and analgesic activity. This method offers a non-invasive alternative for pain management (Deng et al., 2022).
- Innovative Pain Management Solutions: Research on new delivery methods, such as intranasal administration using chitosan nanoparticles, aims to enhance the therapeutic potential and patient compliance of Tapentadol hydrochloride solution for pain management (Javia & Thakkar, 2017).
Informations légales
Produit(s) apparenté(s)
Mention d'avertissement
Danger
Mentions de danger
Conseils de prudence
Classification des risques
Acute Tox. 3 Dermal - Acute Tox. 3 Inhalation - Acute Tox. 3 Oral - Flam. Liq. 2 - STOT SE 1
Organes cibles
Eyes,Central nervous system
Code de la classe de stockage
3 - Flammable liquids
Classe de danger pour l'eau (WGK)
WGK 2
Point d'éclair (°F)
49.5 °F - closed cup
Point d'éclair (°C)
9.7 °C - closed cup
Certificats d'analyse (COA)
Recherchez un Certificats d'analyse (COA) en saisissant le numéro de lot du produit. Les numéros de lot figurent sur l'étiquette du produit après les mots "Lot" ou "Batch".
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Protocoles
-THC solution, 1.0 mg/mL in methanol, ampule of 1 mL, certified reference material
To optimize hydrolysis using β-glucuronidase, factors such as incubation time, temperature, hydrolysis pH, enzyme source, and enzyme concentration must be evaluated for each glucuronide metabolite to be analyzed.
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique