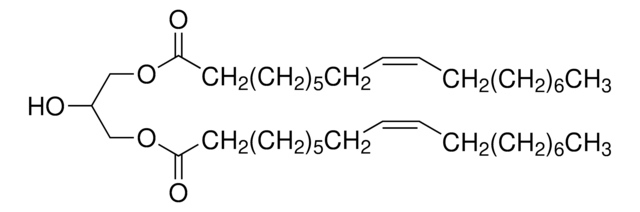800819C
Avanti
18:0-22:6 DG
1-stearoyl-2-docosahexaenoyl-sn-glycerol, chloroform
Synonyme(s) :
1-octadecanoyl-2-(4Z,7Z,10Z,13Z,16Z,19Z-docosahexaenoyl)-sn-glycerol; DG(18:0/22:6(4Z,7Z,10Z,13Z,16Z,19Z)/0:0)
About This Item
Produits recommandés
Pureté
>99% (TLC)
Forme
liquid
Conditionnement
pkg of 1 × 5 mL (800819C-10mg)
Fabricant/nom de marque
Avanti Research™ - A Croda Brand 800819C
Concentration
2 mg/mL (800819C-10mg)
Type de lipide
neutral lipids
neutral glycerides
Conditions d'expédition
dry ice
Température de stockage
−20°C
Catégories apparentées
Description générale
Diacylglycerol mimicks the effects of the tumor-promoting compounds phorbol esters.
Application
- in the preparation of Golgi-like liposomes
- to study its effect on conventional protein kinase C (cPKC) and novel protein kinase C (nPKC) isozymes in vitro
- as a substrate for the measurement of diacylglycerol kinase η1 (DGKη1) activity in vitro
Conditionnement
Stockage et stabilité
Autres remarques
Dry samples of diacylglycerol in chloroform, using a stream of nitrogen. Dissolve the residue in an appropriate volume of ethanol or DMSO, then dilute to the desired aqueous medium.
Most biological responses saturate at 20 to 250 μM sn-1,2-dioctanoylglycerol. Only sn-1,2 isomers appear to be active.
Informations légales
Mention d'avertissement
Danger
Mentions de danger
Classification des risques
Acute Tox. 3 Inhalation - Acute Tox. 4 Oral - Aquatic Chronic 3 - Carc. 2 - Eye Irrit. 2 - Repr. 2 - Skin Irrit. 2 - STOT RE 1 - STOT SE 3
Organes cibles
Central nervous system, Liver,Kidney
Classe de danger pour l'eau (WGK)
WGK 3
Certificats d'analyse (COA)
Recherchez un Certificats d'analyse (COA) en saisissant le numéro de lot du produit. Les numéros de lot figurent sur l'étiquette du produit après les mots "Lot" ou "Batch".
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique