H17082
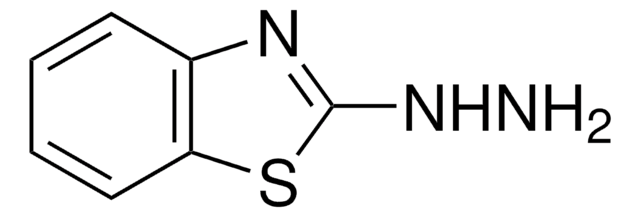
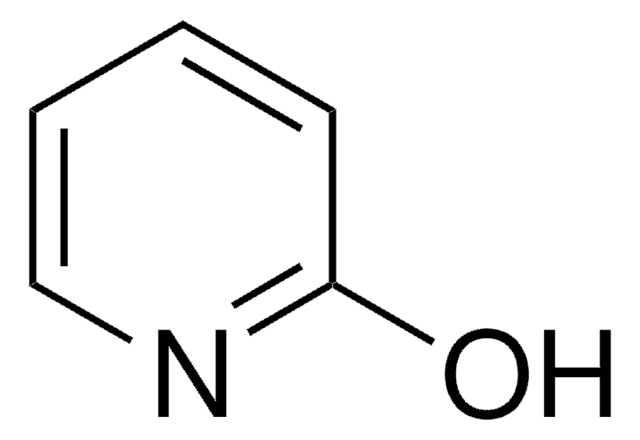
2-Hydrazinopyridine
97%
Synonyme(s) :
2-Pyridylhydrazine
About This Item
Produits recommandés
Niveau de qualité
Pureté
97%
Point d'ébullition
90-92 °C/1 mmHg (lit.)
Pf
41-44 °C (lit.)
Température de stockage
2-8°C
Chaîne SMILES
NNc1ccccn1
InChI
1S/C5H7N3/c6-8-5-3-1-2-4-7-5/h1-4H,6H2,(H,7,8)
Clé InChI
NWELCUKYUCBVKK-UHFFFAOYSA-N
Vous recherchez des produits similaires ? Visite Guide de comparaison des produits
Catégories apparentées
Application
- Enhanced Metal Removal: Utilizing Schiff base functionalized dialdehyde starch, derived from 2-hydrazinopyridine, demonstrated significant potential in enhancing the removal of Cu(II) from solutions. The study included preparation methodologies, performance evaluations, and DFT calculations, showcasing its efficacy in water treatment technologies (Liang et al., 2024).
- Dual Sensing Probe Development: A novel dicyanisophorone-based probe, incorporating 2-hydrazinopyridine, was developed for the dual sensing of Zn(2+) and Cd(2+) via near-infrared fluorescence. This advancement aids in the detection and analysis of heavy metals in various environmental and biological samples (Yan et al., 2023).
- Active Site Analysis in Lysyl Oxidase: The study provided insights into the spatial arrangement of active site components in Lysyl Oxidase-like 2, including the role of 2-hydrazinopyridine, which is critical for understanding the enzyme′s mechanism and potential therapeutic applications (Meier et al., 2022).
- Structural Analysis of Lysyl Oxidase: Research focused on the predicted 3D structure of the amine oxidase domain of Lysyl Oxidase-Like 2, exploring the interaction dynamics facilitated by 2-hydrazinopyridine. This contributes significantly to the field of molecular biology and enzyme function analysis (Meier et al., 2022).
Mention d'avertissement
Warning
Mentions de danger
Conseils de prudence
Classification des risques
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Organes cibles
Respiratory system
Code de la classe de stockage
11 - Combustible Solids
Classe de danger pour l'eau (WGK)
WGK 3
Point d'éclair (°F)
230.0 °F - closed cup
Point d'éclair (°C)
110 °C - closed cup
Équipement de protection individuelle
dust mask type N95 (US), Eyeshields, Gloves
Faites votre choix parmi les versions les plus récentes :
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique