D223204
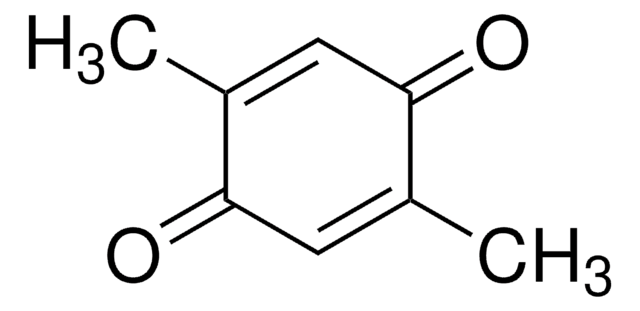
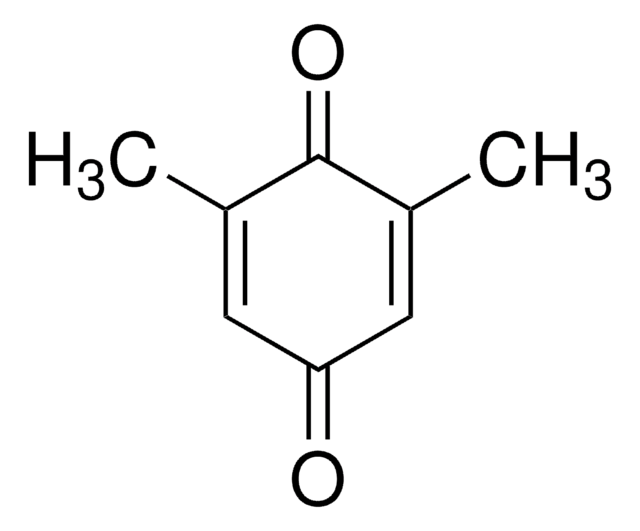
Duroquinone
97%
Synonyme(s) :
2,3,5,6-Tetramethyl-1,4-benzoquinone, Tetramethyl-p-benzoquinone
About This Item
Produits recommandés
Essai
97%
Forme
powder
Pf
110-112 °C (lit.)
Chaîne SMILES
CC1=C(C)C(=O)C(C)=C(C)C1=O
InChI
1S/C10H12O2/c1-5-6(2)10(12)8(4)7(3)9(5)11/h1-4H3
Clé InChI
WAMKWBHYPYBEJY-UHFFFAOYSA-N
Vous recherchez des produits similaires ? Visite Guide de comparaison des produits
Catégories apparentées
Description générale
Application
- Role of Duroquinone in Photosynthetic Research: A study on purple bacterial photosynthetic reaction centers highlighted Duroquinone′s role when incorporated into the QA binding site, impacting the isotope edited FTIR difference spectra, crucial for understanding energy conversion processes in photosynthesis (Zhao et al., 2013).
- Duroquinone as a Molecular Ion Source: Research demonstrated the generation of molecular negative ions by Duroquinone, showcasing its potential in mass spectrometry applications for studying molecular ionization and longevity (Khvostenko et al., 2012).
- Duroquinone in Biochemical Sensors: Investigation into the optimization of gold electrode surfaces with photosystem II monolayers revealed Duroquinone′s critical role in enhancing sensor responses, applicable in biochemical sensor technology (Maly et al., 2005).
- Cytotoxicity of Duroquinone Congeners: A comparative study of 14 p-benzoquinone congeners, including Duroquinone, used quantitative structure-toxicity relationships to evaluate cytotoxicity in rat hepatocytes and PC12 cells, relevant for safety assessments in chemical manufacturing (Siraki et al., 2004).
Code de la classe de stockage
11 - Combustible Solids
Classe de danger pour l'eau (WGK)
WGK 3
Point d'éclair (°F)
Not applicable
Point d'éclair (°C)
Not applicable
Équipement de protection individuelle
dust mask type N95 (US), Eyeshields, Gloves
Faites votre choix parmi les versions les plus récentes :
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Les clients ont également consulté
Global Trade Item Number
| Référence | GTIN |
|---|---|
| D223204-25G | |
| D223204-5G | 4061831826548 |
| D223204-1G | 4061825597713 |
| D223204-500G |
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique