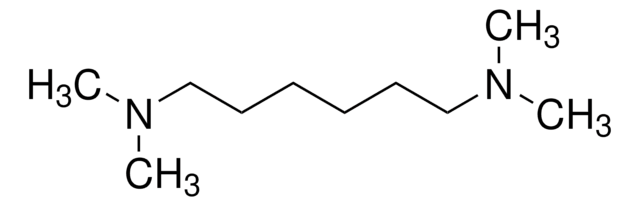
549983
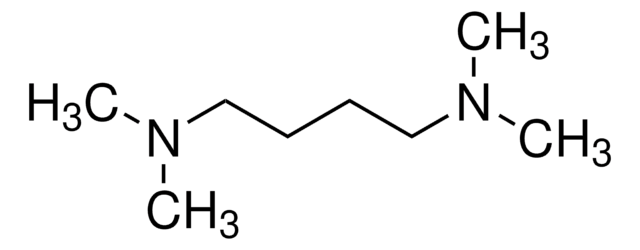
N,N,N′,N′-Tetramethyl-1,3-propanediamine
≥99%
Synonyme(s) :
1,3-Bis(dimethylamino)propane, TMPDA
About This Item
Produits recommandés
Pression de vapeur
760 mmHg ( 145 °C)
Niveau de qualité
Pureté
≥99%
Indice de réfraction
n20/D 1.4234 (lit.)
Point d'ébullition
145-146 °C (lit.)
Densité
0.779 g/mL at 25 °C (lit.)
Groupe fonctionnel
amine
Chaîne SMILES
CN(C)CCCN(C)C
InChI
1S/C7H18N2/c1-8(2)6-5-7-9(3)4/h5-7H2,1-4H3
Clé InChI
DMQSHEKGGUOYJS-UHFFFAOYSA-N
Informations sur le gène
rat ... Grin2a(24409)
Vous recherchez des produits similaires ? Visite Guide de comparaison des produits
Description générale
- As a catalyst for the Baylis-Hillman reaction of cycloalkenones.
- In the preparation of homodimeric asymmetric monomethine cyanine dyes during the bisquaternization process.
- As a ligand (L) for the preparation of dinuclear μ-carbonato-dicopper(II) species.
Application
- Deaggregation of Zinc Dihydride by Lewis Acids Including Carbon Dioxide in the Presence of Nitrogen Donors.: This study explores the deaggregation of zinc dihydride using Lewis acids such as carbon dioxide in the presence of nitrogen donors, including N,N,N′,N′-Tetramethyl-1,3-propanediamine, highlighting its application in organic synthesis and catalyst development (Ritter et al., 2021).
- Contribution of Cross-Linker and Silica Morphology on Cr(VI) Sorption Performances of Organic Anion Exchangers Embedded into Silica Pores.: This research focuses on the sorption performances of Cr(VI) using organic anion exchangers embedded in silica pores, demonstrating the role of N,N,N′,N′-Tetramethyl-1,3-propanediamine in enhancing cross-linking and silica morphology for improved sorption efficiency (Dragan & Humelnicu, 2020).
- Nanopore-induced host-guest charge transfer phenomena in a metal-organic framework.: The study investigates host-guest charge transfer phenomena within nanopores of a metal-organic framework, using N,N,N′,N′-Tetramethyl-1,3-propanediamine as a key component in the framework′s design, demonstrating its significance in materials science and nanoengineering (Yamamoto et al., 2018).
- Cross-Linking of a Hydrophilic, Antimicrobial Polycation toward a Fast-Swelling, Antimicrobial Superabsorber and Interpenetrating Hydrogel Networks with Long Lasting Antimicrobial Properties.: This paper details the development of a hydrophilic, antimicrobial polycation cross-linked with N,N,N′,N′-Tetramethyl-1,3-propanediamine to create a fast-swelling, antimicrobial superabsorber and hydrogel network with prolonged antimicrobial effects (Strassburg et al., 2017).
- CO(2)-Switchable microemulsion based on a pseudogemini surfactant.: The research presents a CO2-switchable microemulsion system based on a pseudogemini surfactant incorporating N,N,N′,N′-Tetramethyl-1,3-propanediamine, showcasing its potential in responsive material systems for environmental applications (Liu et al., 2017).
Informations légales
Mention d'avertissement
Danger
Mentions de danger
Classification des risques
Acute Tox. 3 Inhalation - Acute Tox. 4 Dermal - Acute Tox. 4 Oral - Aquatic Chronic 2 - Eye Dam. 1 - Flam. Liq. 3 - Skin Corr. 1B - STOT SE 3
Organes cibles
Respiratory system
Code de la classe de stockage
3 - Flammable liquids
Classe de danger pour l'eau (WGK)
WGK 2
Point d'éclair (°F)
87.8 °F - Equilibrium method
Point d'éclair (°C)
31 °C - Equilibrium method
Équipement de protection individuelle
Faceshields, Gloves, Goggles, type ABEK (EN14387) respirator filter
Faites votre choix parmi les versions les plus récentes :
Certificats d'analyse (COA)
Vous ne trouvez pas la bonne version ?
Si vous avez besoin d'une version particulière, vous pouvez rechercher un certificat spécifique par le numéro de lot.
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Les clients ont également consulté
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique

![Bis[2-(N,N-dimethylamino)ethyl] ether 97%](/deepweb/assets/sigmaaldrich/product/structures/372/323/505a46ae-b067-4177-8e5f-19a3f4ef9c44/640/505a46ae-b067-4177-8e5f-19a3f4ef9c44.png)