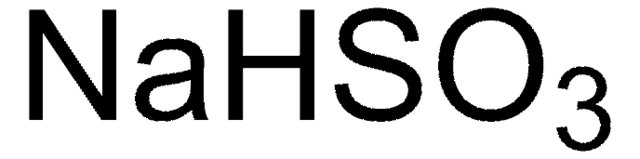
455849
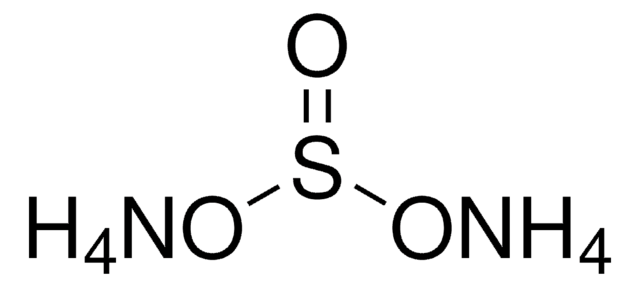
Ammonium hydrogensulfate
99.99% trace metals basis
Synonyme(s) :
Ammonium bisulfate, Ammonium sulfate monobasic
About This Item
Produits recommandés
Niveau de qualité
Essai
99.99% trace metals basis
Forme
crystalline
Impuretés
≤200 mg/kg Trace metallic impurities analysis (ICP)
pb
350 °C (dec.)(lit.)
Pf
121-145 °C (lit.)
Densité
1.79 g/mL at 25 °C (lit.)
Chaîne SMILES
N.OS(O)(=O)=O
InChI
1S/H3N.H2O4S/c;1-5(2,3)4/h1H3;(H2,1,2,3,4)
Clé InChI
BIGPRXCJEDHCLP-UHFFFAOYSA-N
Vous recherchez des produits similaires ? Visite Guide de comparaison des produits
Application
- Enhancement of Aesthetic Dental CAD-CAM Materials through Surface Etching with a Mixed Aqueous Solution of Ammonium Fluoride and Ammonium Hydrogen Sulfate - This study explores the potential of ammonium hydrogen sulfate in surface etching applications for dental materials, focusing on its low toxicity and effective etching capabilities (Y Nishizawa et al., 2024).
- Thermodynamics of ammonioalunite precipitation in ammonium aluminum sulfate solution - Investigates the thermodynamic properties of ammonium aluminum sulfate solutions, providing insights into chemical processes involving ammonium hydrogen sulfate (X Yang et al., 2020).
- Hygroscopic behavior and chemical composition evolution of internally mixed aerosols composed of oxalic acid and ammonium sulfate - Studies the hygroscopic properties of mixed aerosol particles, including those formed with ammonium hydrogen sulfate, to understand atmospheric chemical processes better (X Wang et al., 2017).
Mention d'avertissement
Danger
Mentions de danger
Classification des risques
Eye Dam. 1 - Skin Corr. 1B
Code de la classe de stockage
8B - Non-combustible corrosive hazardous materials
Classe de danger pour l'eau (WGK)
WGK 1
Point d'éclair (°F)
Not applicable
Point d'éclair (°C)
Not applicable
Équipement de protection individuelle
Eyeshields, Faceshields, Gloves, type P3 (EN 143) respirator cartridges
Faites votre choix parmi les versions les plus récentes :
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Les clients ont également consulté
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique