390704
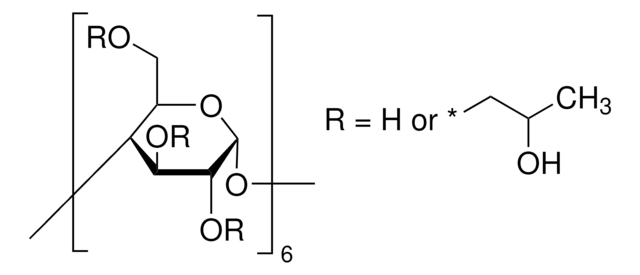
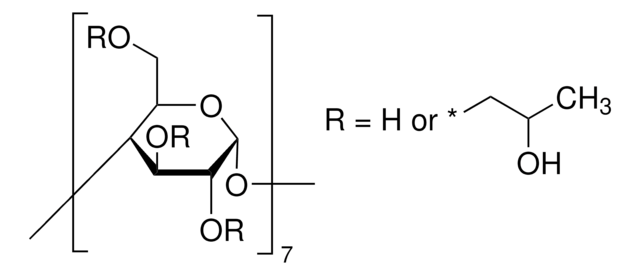
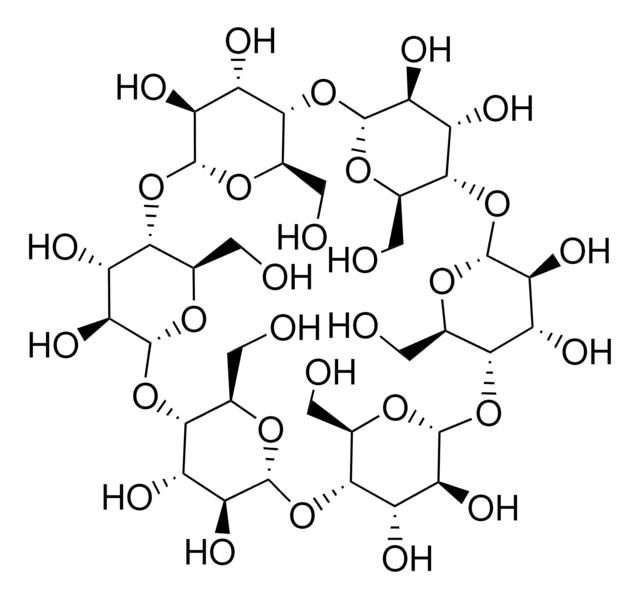
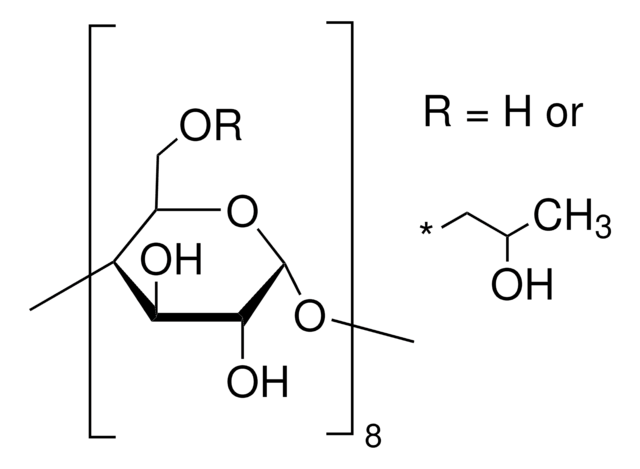
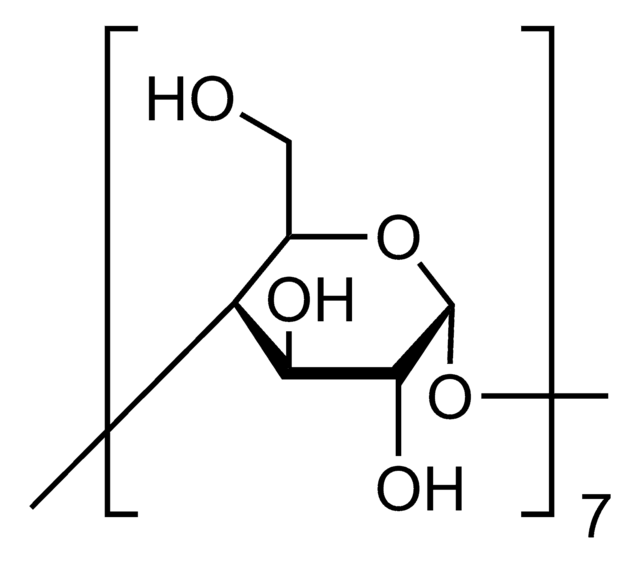
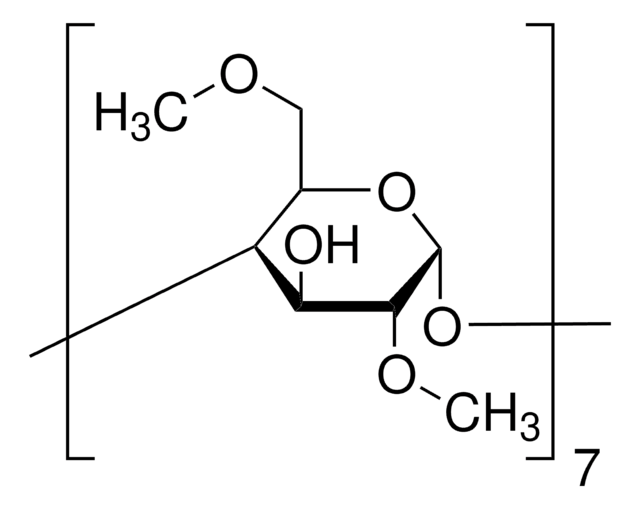
(2-Hydroxypropyl)-γ-cyclodextrin
extent of labeling: 0.6 molar substitution
Synonyme(s) :
HP-γ-CD, HPGCD, HGC
About This Item
Produits recommandés
Forme
powder
Niveau de qualité
Activité optique
[α]20/D +145°, c = 1 in H2O
Poids mol.
average Mw ~1,580
Ampleur du marquage
0.6 molar substitution
InChI
1S/C51H88O38/c1-14(56)8-73-11-21-42-29(64)36(71)50(81-21)85-40-19(6-54)79-48(34(69)27(40)62)89-44-23(13-75-10-16(3)58)82-51(37(72)30(44)65)86-41-20(7-55)78-47(33(68)26(41)61)88-43-22(12-74-9-15(2)57)80-49(35(70)28(43)63)84-39-18(5-53)76-45(31(66)24(39)59)83-38-17(4-52)77-46(87-42)32(67)25(38)60/h14-72H,4-13H2,1-3H3/t14?,15?,16?,17-,18-,19-,20-,21-,22-,23-,24-,25-,26-,27-,28-,29-,30-,31-,32-,33-,34-,35-,36-,37-,38-,39-,40-,41-,42-,43-,44-,45-,46-,47-,48-,49-,50-,51-/m0/s1
Clé InChI
ODLHGICHYURWBS-RYJYQAAZSA-N
Vous recherchez des produits similaires ? Visite Guide de comparaison des produits
Description générale
Application
- As a mobile phase additive in the study of the host-guest interaction with organic low molecular mass compounds prior to their quantification using reversed phase-high performance liquid chromatography (RP-HPLC) technique.
- As a chiral surfactant for the analysis of econazole by micellar electrokinetic chromatography (MEKC).
- As an analytical standard for the determination of the analyte in biological samples by HPLC.
- As a chiral selector for the identification of propiconazole by MEKC.
Code de la classe de stockage
11 - Combustible Solids
Classe de danger pour l'eau (WGK)
WGK 3
Point d'éclair (°F)
Not applicable
Point d'éclair (°C)
Not applicable
Équipement de protection individuelle
Eyeshields, Gloves, type N95 (US)
Certificats d'analyse (COA)
Recherchez un Certificats d'analyse (COA) en saisissant le numéro de lot du produit. Les numéros de lot figurent sur l'étiquette du produit après les mots "Lot" ou "Batch".
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Les clients ont également consulté
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique