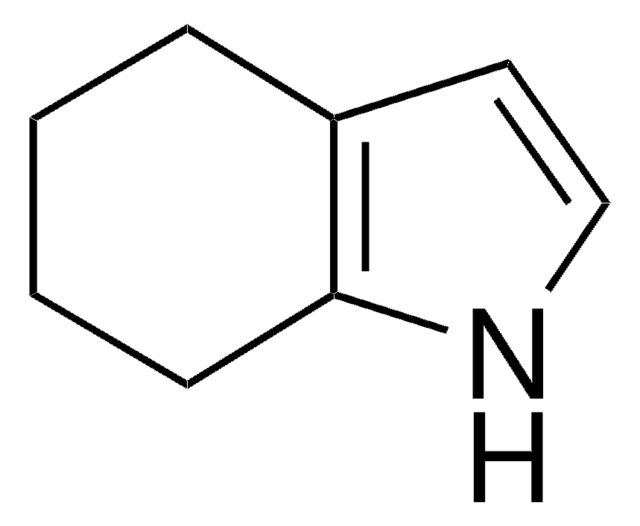
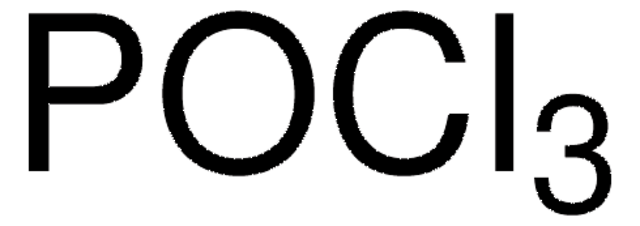357839
1,5,6,7-Tetrahydro-4H-indol-4-one
98%
Synonyme(s) :
4,5,6,7-Tetrahydro-4-oxoindole, NSC 131681
Se connecterpour consulter vos tarifs contractuels et ceux de votre entreprise/organisme
About This Item
Formule empirique (notation de Hill) :
C8H9NO
Numéro CAS:
Poids moléculaire :
135.16
Numéro MDL:
Code UNSPSC :
12352100
ID de substance PubChem :
Nomenclature NACRES :
NA.22
Produits recommandés
Essai
98%
Pf
188-190 °C (lit.)
Groupe fonctionnel
ketone
Chaîne SMILES
O=C1CCCc2[nH]ccc12
InChI
1S/C8H9NO/c10-8-3-1-2-7-6(8)4-5-9-7/h4-5,9H,1-3H2
Clé InChI
KASJZXHXXNEULX-UHFFFAOYSA-N
Description générale
Iodination of 1,5,6,7-tetrahydro-4H-indol-4-one using 1-chloromethyl-4-fluoro-1,4-diazoniabicyclo[2.2.2]octane bis(tetrafluoroborate) yields α-iodo derivative as the main product.
Application
1,5,6,7-Tetrahydro-4H-indol-4-one may be used in the preparation of:
- 4-oxo-4,5,6,7-tetrahydro-1H-indole-2-carbonitrile
- methyl 2-(4-oxo-4,5,6,7-tetrahydro-1H-indol-1-yl)acrylate
- 3-(4-oxo-4,5,6,7-tetrahydro-1H-indol-1-yl)propanenitrile
- potent and orally active 5-HT1A agonists, (R)-(+)- and (S)-(-)-1-formyl-6,7,8,9-tetrahydro-N,N-dipropyl-3H-benz[e]indol-8-amines
- Reactant in synthesis of psammopemmin A as antitumor agent
- Reactant in synthesis of a 1,3,4,5-tetrahydrobenzindole β-ketoesters and tricyclic tetrahydrobenzindoles via C-H insertion reactions
- Reactant in preparation of tricyclic indole and dihydroindole derivatives as inhibitors of guanylate cyclase
- Reactant in preparation of condensed pyrroloindoles via Pd-catalyzed intramolecular C-H bond functionalization of (halobenzyl)pyrroles
- Reactant in enantioselective preparation of arylalkenyl indoles via asymmetric C-H insertion of rhodium carbenoids followed by Cope rearrangement-elimination
Mention d'avertissement
Warning
Mentions de danger
Conseils de prudence
Classification des risques
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Organes cibles
Respiratory system
Code de la classe de stockage
11 - Combustible Solids
Classe de danger pour l'eau (WGK)
WGK 3
Équipement de protection individuelle
dust mask type N95 (US), Eyeshields, Gloves
Faites votre choix parmi les versions les plus récentes :
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
DNA binders: 1. evaluation of DNA-interactive ability, design, and synthesis of novel intercalating agents
Sechi M, et al.
Letters in Drug Design & Discovery, 6(1), 56-62 (2009)
Direct α-iodination of aryl alkyl ketones by elemental iodine activated by 1-chloromethyl-4-fluoro-1, 4-diazoniabicyclo [2.2. 2] octane bis (tetrafluoroborate).
Jereb M, et al.
Synthesis, 06, 0853-0858 (2003)
Synthesis of pyrrolo [1, 2-a] indole-1, 8 (5H)-diones as new synthons for developing novel tricyclic compounds of pharmaceutical interest.
Sechi M, et al.
ARKIVOC (Gainesville, FL, United States), 5, 97-106 (2004)
Synthesis of (R)-and (S)-1-formyl-6, 7, 8, 9-tetrahydro-N, N-(dipropyl)-3H-benz [e] indol-8-amines: potent and orally active 5-HT1A receptor agonists.
Lin C-H, et al.
Journal of Heterocyclic Chemistry, 31(1), 129-139 (1994)
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique