326755
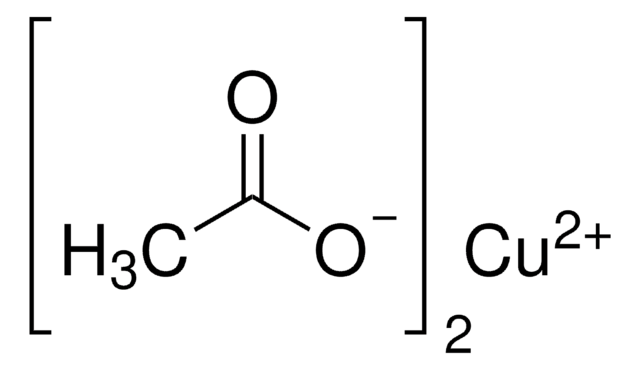
Copper(II) acetate
98%
Synonyme(s) :
Cupric acetate
About This Item
Produits recommandés
Densité de vapeur
6.9 (vs air)
Niveau de qualité
Pureté
98%
Forme
powder or crystals
Capacité de réaction
reaction type: click chemistry
Caractéristiques du produit alternatif plus écologique
Catalysis
Learn more about the Principles of Green Chemistry.
sustainability
Greener Alternative Product
Autre catégorie plus écologique
, Aligned
Chaîne SMILES
CC(=O)O[Cu]OC(C)=O
InChI
1S/2C2H4O2.Cu/c2*1-2(3)4;/h2*1H3,(H,3,4);/q;;+2/p-2
Clé InChI
OPQARKPSCNTWTJ-UHFFFAOYSA-L
Vous recherchez des produits similaires ? Visite Guide de comparaison des produits
Description générale
Copper(II) acetate also known as cupric acetate, can be used as a catalyst in various processes in the field of greener chemistry. It is particularly useful in cross-coupling reactions, where it can promote the formation of carbon-carbon or carbon-heteroatom bonds, without the need for hazardous reagents or solvents
Application
Copper-catalyzed reductive amination of aromatic and aliphatic ketones with anilines using environmental-friendly molecular hydrogen
Copper(II) acetate is used as a catalyst:
- In the N-arylation of α-amino esters with p-tolylboronic acid to synthesize biaryls via cross-coupling reactions
- In the the synthesis of substituted isoxazole derivatives
Mention d'avertissement
Danger
Mentions de danger
Conseils de prudence
Classification des risques
Acute Tox. 4 Oral - Aquatic Acute 1 - Aquatic Chronic 2 - Eye Dam. 1 - Skin Corr. 1B
Code de la classe de stockage
8B - Non-combustible corrosive hazardous materials
Classe de danger pour l'eau (WGK)
WGK 3
Point d'éclair (°F)
does not flash
Point d'éclair (°C)
does not flash
Équipement de protection individuelle
dust mask type N95 (US), Eyeshields, Gloves
Faites votre choix parmi les versions les plus récentes :
Certificats d'analyse (COA)
Vous ne trouvez pas la bonne version ?
Si vous avez besoin d'une version particulière, vous pouvez rechercher un certificat spécifique par le numéro de lot.
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Les clients ont également consulté
Articles
In this article, we will discuss coinage metal deposition processes in order to provide a sense of the most critical precursors, reducing agents, and processes.
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique