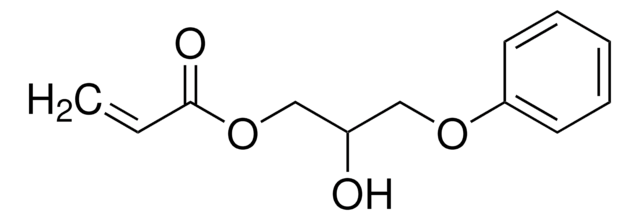
292818
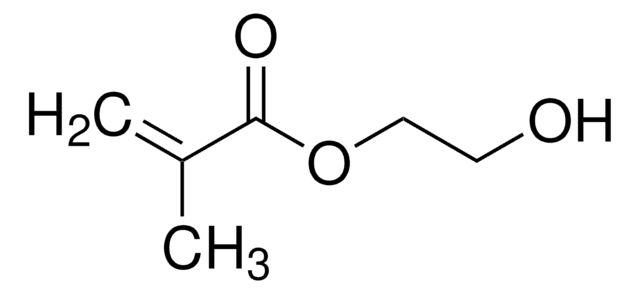
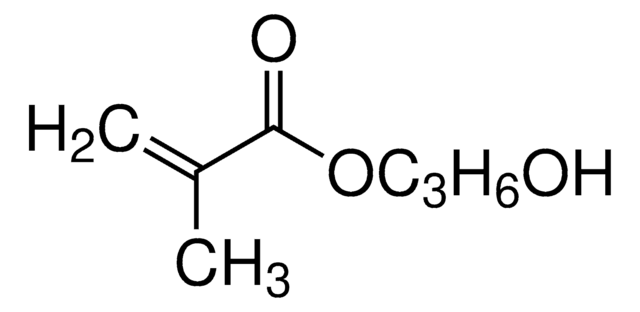
2-Hydroxyethyl acrylate
96%, contains 200-650 ppm monomethyl ether hydroquinone as inhibitor
Synonyme(s) :
Ethylene glycol monoacrylate
About This Item
Produits recommandés
Densité de vapeur
>1 (vs air)
Niveau de qualité
Pression de vapeur
<0.1 mmHg ( 20 °C)
Pureté
96%
Forme
solid
Contient
200-650 ppm monomethyl ether hydroquinone as inhibitor
Indice de réfraction
n20/D 1.45 (lit.)
Point d'ébullition
90-92 °C/12 mmHg (lit.)
Densité
1.011 g/mL at 25 °C (lit.)
Température de stockage
2-8°C
Chaîne SMILES
OCCOC(=O)C=C
InChI
1S/C5H8O3/c1-2-5(7)8-4-3-6/h2,6H,1,3-4H2
Clé InChI
OMIGHNLMNHATMP-UHFFFAOYSA-N
Vous recherchez des produits similaires ? Visite Guide de comparaison des produits
Description générale
Application
Mention d'avertissement
Danger
Mentions de danger
Conseils de prudence
Classification des risques
Acute Tox. 3 Dermal - Acute Tox. 4 Oral - Aquatic Acute 1 - Aquatic Chronic 3 - Eye Dam. 1 - Skin Corr. 1B - Skin Sens. 1
Code de la classe de stockage
6.1A - Combustible acute toxic Cat. 1 and 2 / very toxic hazardous materials
Classe de danger pour l'eau (WGK)
WGK 3
Point d'éclair (°F)
213.8 °F - closed cup
Point d'éclair (°C)
101 °C - closed cup
Équipement de protection individuelle
Faceshields, Gloves, Goggles, type ABEK (EN14387) respirator filter
Faites votre choix parmi les versions les plus récentes :
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Les clients ont également consulté
Articles
The manufacture of monomers for use in ophthalmic applications is driven by the need for higher purity, improved reliability of manufacturing supply, but ultimately by the need for the increased comfort, convenience, and safety of contact lens wearers. Daily wear contact lenses have the potential to fill this need for many customers; however, their widespread use is constrained by higher costs compared to weekly- or monthly-based lenses. New approaches that improve cost structure and result in higher quality raw materials are needed to help make contact lenses more affordable and accelerate growth of the contact lens market.
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique