217964
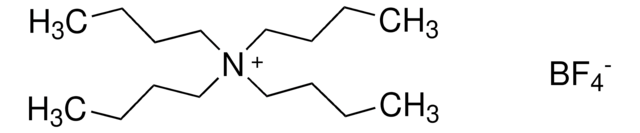
Tetrabutylammonium tetrafluoroborate
99%
Synonyme(s) :
Ammonium tetra-n-butyl tetrafluoroborate
About This Item
Produits recommandés
Niveau de qualité
Essai
99%
Forme
powder
Pf
155-161 °C (lit.)
Solubilité
methanol: soluble 10%, clear, colorless
Chaîne SMILES
F[B-](F)(F)F.CCCC[N+](CCCC)(CCCC)CCCC
InChI
1S/C16H36N.BF4/c1-5-9-13-17(14-10-6-2,15-11-7-3)16-12-8-4;2-1(3,4)5/h5-16H2,1-4H3;/q+1;-1
Clé InChI
NNZZSJSQYOFZAM-UHFFFAOYSA-N
Vous recherchez des produits similaires ? Visite Guide de comparaison des produits
Description générale
Tetrabutylammonium tetrafluoroborate (TBATFB) is a phase transfer catalyst. It can be synthesized by the reaction between 30% aqueous solution of tetrafluoroboric acid and 40% aqueous solution of tetrabutylamonium hydroxide. Tetrabutylammonium tetrafluoroborate acts as an electrolyte and inhibits the self-assembly of alkylthiosulfate on gold.
Application
- As supporting electrolyte in the voltammetric determination of Δ(9)-tetrahydrocannabinol (Δ(9)-THC).
- Synthesis of biologically relevant macrolactones, Sansalvamide A.
- As supporting electrolyte in the determination of the oxidation and reduction potentials of 5,10,15,20-tetra[3-(3-trifluoromethyl)phenoxy]porphyrin by cyclic voltammetry.
- Preparation of 1:1 adduct with 1,10-phenanthroline.
- Used to prepare other tetrabutylammonium salts in aqueous solutions.
- As electrolyte additive in the synthesis of conducting poly(thiophenes).
Mention d'avertissement
Danger
Mentions de danger
Conseils de prudence
Classification des risques
Acute Tox. 4 Oral - Eye Dam. 1 - Skin Irrit. 2 - STOT SE 3
Organes cibles
Respiratory system
Code de la classe de stockage
11 - Combustible Solids
Classe de danger pour l'eau (WGK)
WGK 3
Point d'éclair (°F)
No data available
Point d'éclair (°C)
No data available
Équipement de protection individuelle
dust mask type N95 (US), Eyeshields, Gloves
Faites votre choix parmi les versions les plus récentes :
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Les clients ont également consulté
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique