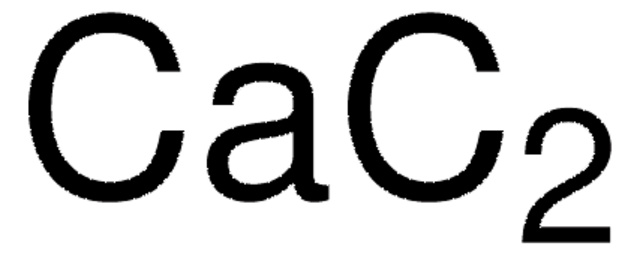21039
Calcium carbide
granulated, technical, ≥75% (gas-volumetric)
Synonyme(s) :
Calcium acetylide, Calcium dicarbide
About This Item
Produits recommandés
Qualité
technical
granulated
Niveau de qualité
Pureté
≥75% (gas-volumetric)
Forme
pieces
Taille des particules
0.1-1 mm
Densité
2.22 g/mL at 25 °C (lit.)
Chaîne SMILES
[Ca++].[C-]#[C-]
InChI
1S/C2.Ca/c1-2;/q-2;+2
Clé InChI
UIXRSLJINYRGFQ-UHFFFAOYSA-N
Vous recherchez des produits similaires ? Visite Guide de comparaison des produits
Description générale
Application
- NUCLEOPHILIC ADDITION OF PHOSPHINOXIDES AND ALCOHOLS TO ACETYLENE GENERATED in situ FROM CALCIUM CARBIDE: This study discusses the reactivity of acetylene generated from calcium carbide for various organic synthesis applications (Aleksandrovna, 2024).
- Construction of 3-Methyl-2-Substituted Benzofurans and 3-Methyl-2-Substituted Benzothiophenes Using Solid Calcium Carbide as a Substitute for Gaseous …: Describes a synthetic method using solid calcium carbide in organic synthesis, highlighting its practicality and environmental benefits (Wang et al., 2024).
Mention d'avertissement
Danger
Mentions de danger
Conseils de prudence
Classification des risques
Eye Dam. 1 - Skin Irrit. 2 - STOT SE 3 - Water-react 1
Organes cibles
Respiratory system
Code de la classe de stockage
4.3 - Hazardous materials which set free flammable gases upon contact with water
Classe de danger pour l'eau (WGK)
WGK 1
Point d'éclair (°F)
Not applicable
Point d'éclair (°C)
Not applicable
Équipement de protection individuelle
Eyeshields, Gloves, type P3 (EN 143) respirator cartridges
Faites votre choix parmi les versions les plus récentes :
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Les clients ont également consulté
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique