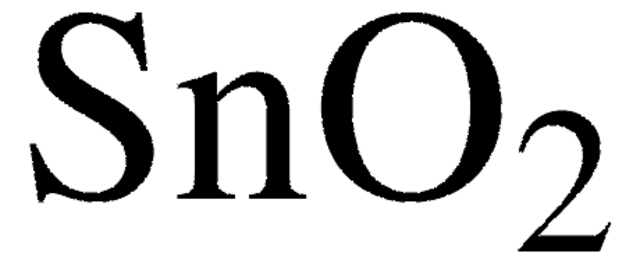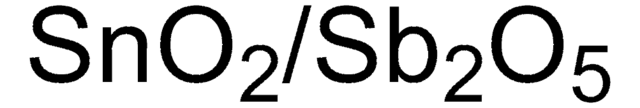204714
Tin(IV) oxide
≥99.99% trace metals basis
Synonyme(s) :
Stannic oxide
Se connecterpour consulter vos tarifs contractuels et ceux de votre entreprise/organisme
About This Item
Formule linéaire :
SnO2
Numéro CAS:
Poids moléculaire :
150.71
Numéro CE :
Numéro MDL:
Code UNSPSC :
12352303
eCl@ss :
38140208
ID de substance PubChem :
Nomenclature NACRES :
NA.23
Produits recommandés
Niveau de qualité
Essai
≥99.99% trace metals basis
Forme
powder and chunks
Densité
6.95 g/mL at 25 °C (lit.)
Application(s)
battery manufacturing
Chaîne SMILES
O=[Sn]=O
InChI
1S/2O.Sn
Clé InChI
XOLBLPGZBRYERU-UHFFFAOYSA-N
Vous recherchez des produits similaires ? Visite Guide de comparaison des produits
Description générale
Tin(IV) oxide, also known as stannic oxide, is a yellow-green powder that crystallizes in the rutile structure. It is a wide bandgap (3.6 eV) semiconductor with high transparency in the visible range of the electromagnetic spectrum and relatively high electronic conductivity. Its chemical stability and high purity of ≥99.99% trace metals basis make it suitable for use in demanding conditions, such as semiconductor and biomedical applications, where it is widely used in medical imaging devices, biosensors, and diagnostic tools. It is also utilized in battery technologies, including lithium-ion batteries, as a conversion-type anode material due to its high energy storage capacity and stability and a precursor for making tin compounds and complex metal oxides.
Application
- Fluorinated Cation-Based 2D Perovskites for Efficient and Stable 3D/2D Heterojunction Perovskite Solar Cells.: This research explores the application of tin(IV) oxide in creating efficient and stable perovskite solar cells, focusing on the improvement of the solar cells′ overall performance (Shaw PE et al., 2023).
- Tin(IV) Oxide Electron Transport Layer via Industrial-Scale Pulsed Laser Deposition for Planar Perovskite Solar Cells.: The study discusses the use of tin(IV) oxide as an electron transport layer applied through industrial-scale pulsed laser deposition, enhancing the functionality and efficiency of planar perovskite solar cells (Bolink HJ et al., 2023).
- Periodic Acid Modification of Chemical-Bath Deposited SnO2 Electron Transport Layers for Perovskite Solar Cells and Mini Modules.: This paper presents a methodology for the modification of SnO2 electron transport layers, used to increase the efficiency of perovskite solar cells and mini-modules (Lin H et al., 2023).
- Zwitterion-Functionalized SnO2 Substrate Induced Sequential Deposition of Black-Phase FAPbI3 with Rearranged PbI2 Residue.: Research on enhancing the deposition of black-phase FAPbI3 on zwitterion-functionalized SnO2 substrates, focusing on perovskite solar cell improvements (Zhao Y et al., 2022).
- Improved stability and efficiency of polymer-based selenium solar cells through the usage of tin(iv) oxide in the electron transport layers and the analysis of aging dynamics.: The study investigates the role of tin(IV) oxide in enhancing the stability and efficiency of polymer-based selenium solar cells (Zhang Q et al., 2020).
Code de la classe de stockage
11 - Combustible Solids
Classe de danger pour l'eau (WGK)
nwg
Point d'éclair (°F)
Not applicable
Point d'éclair (°C)
Not applicable
Équipement de protection individuelle
Eyeshields, Gloves, type N95 (US)
Faites votre choix parmi les versions les plus récentes :
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Les clients ont également consulté
Gun-Joo Sun et al.
Nanotechnology, 24(2), 025504-025504 (2012-12-15)
Networked SnO(2) nanowire sensors were achieved using the selective growth of SnO(2) nanowires and their tangling ability, particularly on on-chip V-groove structures, in an effort to overcome the disadvantages imposed on the conventional trench-structured SnO(2) nanowire sensors. The sensing performance
Li-Ping Li et al.
Chemical communications (Cambridge, England), 49(17), 1762-1764 (2013-01-25)
ZnSn(OH)(6) and binary-component SnO(2)-ZnSn(OH)(6) were introduced as affinity probes for phosphopeptide enrichment for the first time. Two strategies, either ZnSn(OH)(6) and SnO(2) serial enrichment or binary-component SnO(2)-ZnSn(OH)(6) enrichment in a single run, were proposed to enhance multi-phosphopeptide enrichment and to
Dawei Su et al.
Chemical communications (Cambridge, England), 49(30), 3131-3133 (2013-03-13)
An in situ hydrothermal synthesis approach has been developed to prepare SnO2@graphene nanocomposites. The nanocomposites exhibited a high reversible sodium storage capacity of above 700 mA h g(-1) and excellent cyclability for Na-ion batteries. In particular, they also demonstrated a
Linlin Li et al.
Nanoscale, 5(1), 134-138 (2012-11-14)
Novel eggroll-like CaSnO(3) nanotubes have been prepared by a single spinneret electrospinning method followed by calcination in air for the first time. The electrospun sample as a lithium-ion battery electrode material exhibited improved cycling stability and rate capability by virtue
Yinzhu Jiang et al.
ACS applied materials & interfaces, 4(11), 6216-6220 (2012-10-31)
Porous SnO₂/graphene composite thin films are prepared as anodes for lithium ion batteries by the electrostatic spray deposition technique. Reticular-structured SnO₂ is formed on both the nickel foam substrate and the surface of graphene sheets according to the scanning electron
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique