139262
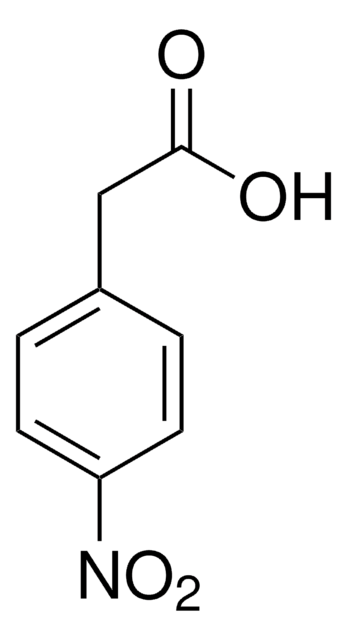
4-Chlorophenylacetic acid
ReagentPlus®, 99%
About This Item
Produits recommandés
Niveau de qualité
Gamme de produits
ReagentPlus®
Pureté
99%
Pf
102-105 °C (lit.)
Solubilité
ethanol: soluble 100 mg/mL, clear, faintly yellow
Groupe fonctionnel
carboxylic acid
chloro
Chaîne SMILES
OC(=O)Cc1ccc(Cl)cc1
InChI
1S/C8H7ClO2/c9-7-3-1-6(2-4-7)5-8(10)11/h1-4H,5H2,(H,10,11)
Clé InChI
CDPKJZJVTHSESZ-UHFFFAOYSA-N
Vous recherchez des produits similaires ? Visite Guide de comparaison des produits
Catégories apparentées
Description générale
Application
Informations légales
Mention d'avertissement
Warning
Mentions de danger
Classification des risques
Acute Tox. 4 Dermal - Acute Tox. 4 Inhalation - Eye Irrit. 2 - Skin Irrit. 2
Code de la classe de stockage
11 - Combustible Solids
Classe de danger pour l'eau (WGK)
WGK 3
Équipement de protection individuelle
dust mask type N95 (US), Eyeshields, Gloves
Faites votre choix parmi les versions les plus récentes :
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Les clients ont également consulté
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique